Abstract
Imiquimod is an immune response modifier currently used as a topical treatment of genital warts, basal cell carcinoma, cutaneous metastasis of malignant melanoma, and vascular tumors. We developed more efficient killers from the same family of compounds that can induce apoptosis without the prominent pro-inflammatory response associated with imiquimod. Among these new products, tk;4EAPB0203, a member of the imidazo[1,2-a]quinoxalines, exhibits an important cytotoxic activity in vitro. HTLV-I–associated adult T-cell leukemia (ATL) and HTLV-I–negative peripheral T-cell lymphomas are associated with poor prognosis. Using potentially achievable concentrations of EAPB0203, we demonstrate inhibition of cell proliferation, G2/M cell- cycle arrest, and induction of apoptosis in HTLV-I–transformed and HTLV-I–negative malignant T cells and fresh ATL cells, whereas normal resting or activated T lymphocytes were resistant. EAPB0203 treatment significantly down-regulated the antiapoptotic proteins c-IAP-1 and Bcl-XL and resulted in a significant loss of mitochondrial membrane potential, cytoplasmic release of cytochrome c, and caspase-dependent apoptosis. Moreover, in HTLV-I–transformed cells only, EAPB0203 treatment stabilized p21 and p53 proteins but had no effect on NF-κB activation. These results support a potential therapeutic role for EAPB0203 in ATL and HTLV-I–negative T-cell lymphomas, either as a systemic or topical therapy for skin lesions.
Introduction
Imiquimod, the first member of the imidazoquinolone family, is an immune response modifier with potent antiviral and antitumor activity in vivo. This product is currently approved as a topical treatment of external genital warts caused by human papilloma virus.1-3 Recent evidence suggests that imiquimod is also efficacious as a topical therapy for basal cell carcinoma, intraepidermal keratinocyte neoplasias, cutaneous metastasis of malignant melanoma, and vascular tumors.4,5 Moreover, imiquimod applied systemically in animal experiments has proven efficacy in a variety of transplantable tumors, including colon carcinomas, melanomas, lung sarcomas, mammary carcinomas, and bladder carcinomas.6-8
It has been shown that imiquimod exerts its antiviral and antitumor effects in vivo, primarily by stimulating both the innate and adaptive immune responses. Imiquimod effects are mediated by the secretion of proinflammatory cytokines, including interferon-α, interferon gamma, interleukin 6, interleukin 12, and tumor necrosis factor-α.9 However, recent in vitro studies showed that imiquimod, at clinically achievable concentrations, directly induces apoptosis in malignant keratinocytes and malignant melanoma cells, in the absence of immune cells. Furthermore, melanoma cell lines derived from imiquimod-resistant cutaneous metastasis, were also resistant to imiquimod in vitro. Interestingly, normal primary human melanocytes are resistant to imiquimod.10
However, because imiquimod induces a significant pro-inflammatory response and stimulates the production of proinflammatory cytokines that can exert deleterious effects, more efficient killers from the same family of compounds, which can induce apoptosis without a prominent proinflammatory response, are needed. In a previous study, we designed and analyzed a series of imidazo[1,2-a]quinoxalines, as possible imiquimod analogues. We found that, contrary to imiquimod, these imidazo[1,2-a]quinoxalines inhibit both the production and the effects of tumor necrosis factor-α; hence, these imiquimod antagonists can be considered as potential anti-inflammatory drugs.11 Among these new products, EAPB0203 exhibits an important cytotoxic activity in vitro and is 50 times more potent than imiquimod against a human melanoma cell line (G.M. et al, unpublished data, 2007).
The retrovirus HTLV-I is the causative agent of adult T-cell leukemia/lymphoma (ATL), an aggressive malignancy of CD4+ T lymphocytes.12 ATL is preceded by oligoclonal expansions of HTLV-I–infected activated T cells13 as a result of the viral transactivator protein Tax expression, which activates various cellular genes including cAMP response element binding protein/ATF, AP-1, and NF-κB, functionally inactivates p53 and interferes with several cell-cycle regulators, including cyclins and cdk inhibitors.14,15
ATL is a peripheral T-cell lymphoma with 4 clinical subtypes: the acute form, the chronic form, the smoldering form, and the ATL lymphoma.16 The acute and lymphomatous types of ATL have a poor prognosis, with a median survival of approximately 6 and 10 months, respectively.17,18 Smoldering and chronic forms have a relatively indolent course and are frequently associated with limited skin lesions. In these indolent forms, conventional chemotherapy is associated with an exacerbation of the cell-mediated immune deficiency and opportunistic infections, with very little benefit on survival, if any. Hence, in patients with smoldering or low-risk chronic ATL, chemotherapy-free treatment strategy using antiretroviral combination, such as zidovudine and interferon-α, is recommended.18 However, there is no available specific therapy for the cutaneous lesions, particularly in otherwise asymptomatic patients who do not require systemic therapy.
In this report, we investigated the effect of potentially achievable concentrations of EAPB0203, on cell growth and apoptosis of HTLV-I–transformed and HTLV-I–negative malignant T cells. Our data demonstrate selective effects on malignant cells and support a potential therapeutic role for EAPB0203 in patients with ATL and other HTLV-I–negative T-cell lymphomas, either as a systemic or topical therapy for skin lesions of ATL or other cutaneous T-cell lymphomas.
Methods
Cells, drugs, and antibodies
The HTLV-I–infected CD4+ T cell lines HuT-102, MT-2, and C91-PL and the HTLV-I–negative malignant CD4+ T cell lines CEM, Jurkat, HuT-78, and MOLT-4 were generously provided by Dr A. Gessain (Pasteur Institute, Paris, France) and were grown as previously described.19 Peripheral blood mononuclear cells (PBMCs) were isolated, after informed consent, from 1 patient with acute ATL, 1 patient with chronic ATL, and 2 healthy HTLV-I–negative donors, using Ficoll-Hypaque (Lymphoprep, Nyegaard, Norway). Cells were cultured for 24 hours at 105 cells/mL in RPMI 1640 medium containing 10% fetal calf serum (Invitrogen, Paisley, United Kingdom) and antibiotics. Activated PBMCs were grown in Ham's F10 medium (Invitrogen) supplemented with 2% phytohemagglutinin (PHA; Invitrogen). Cell growth was assessed by cell count using trypan blue dye exclusion protocols and the CellTiter 96 cell proliferation assay kit (Promega, Madison, WI).
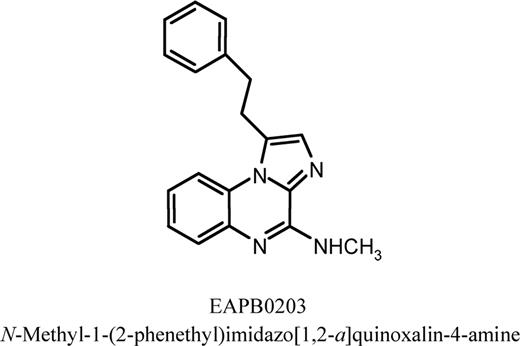
EAPB0203, N-methyl-1-(2-phenethyl)imidazo[1,2-a]quinoxalin-4-amine (Figure 1), was prepared as stock solutions in dimethylsulfoxide (DMSO) at 0.1 M, and stored at −80°C. The final concentration of DMSO never exceeded 0.1%, and this concentration showed no effect on the proliferation of all tested cell lines. The general caspase inhibitor z-VAD (Bachem Bioscience, King of Prussia, PA) was dissolved in DMSO and used at concentration 50 μM. Antibodies p21, bcl-2, bcl-XL, XIAP, c-IAP-1, c-IAP-2, and poly(ADP-ribose) polymerase (PARP) and caspases 3, 8, and 9 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Biogenesis (Stinford Fload, United Kingdom). Rabbit polyclonal anti-p53 was purchased from Chemicon International (Temecula, CA).
Cell-cycle analysis
Cells were harvested, washed twice with cold phosphate-buffered saline, fixed in cold (−20°C) 100% ethanol and kept overnight at 4°C. Subsequently, cells were rinsed with phosphate-buffered saline, treated with Tris-HCl buffer (pH 7.4) containing 1% RNase and stained with propidium iodide (PI) at 100 μg/mL (final concentration). Distribution of cell-cycle phases with different DNA contents was determined using a FACScan flow cytometer (BD Biosciences, San Jose, CA). In each sample, 10 000 ungated events were acquired. Analysis of cell-cycle distribution (including apoptosis) was performed using Cell-Quest software (BD Biosciences).
Apoptosis assays
Annexin V staining.
Cells under study were incubated with EAPB0203. Both phosphatidyl-serine (PS) exposure and viability (permeability) were assessed using annexin V-fluorescein isothiocyanate (FITC) kit (Roche Diagnostics, Mannheim, Germany) according to manufacturer's instructions. Nuclei of cells were counterstained with PI. Cells were analyzed by fluorescent microscopy (200 cells counted for each treatment condition). Apoptosis was estimated by the relative amount of FITC-positive–PI-negative cell populations. Briefly, annexin V conjugated to fluorescein allows the identification of apoptotic cells, whereas PI labels dead cells.
Nuclear staining.
Cells under study were treated with 5 μM EAPB0203 for 24 to 48 hours. Nuclei were labeled with Hoechst 33 342 (Polysciences, Warrington, PA) for 15 minutes at room temperature. Cells were then mounted and observed under fluorescence microscopy using ultraviolet filter pack (200 cells counted for each treatment condition). The total number of nuclei and the percentage of apoptotic nuclei with condensed chromatin were noted.
Measurement of mitochondrial membrane potential
Quantification of mitochondrial membrane potential was determined by rhodamine (R123) retention. R123 is a cationic fluorescent dye that accumulates in active mitochondria with high membrane potentials. EAPB0203-treated cells were washed twice in 130 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 1 mM CaCl2, 1 mM Mgcl2, and 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, pH 7.4, and were then stained with 5μM R123 (Sigma-Aldrich, St Louis, MO) for 30 minutes at 37°C. Cells were washed twice with the former buffer. R123 was excited at 488 nm, and fluorescence emission at 525 nm was assessed using flow cytometry. The results are representative of 2 independent experiments.
Measurement of cytochrome c release
Cytochrome c release was measured using the Cytochrome c ELISA Kit (EMD Biosciences, Darmstadt, Germany). To determine the distribution of cytochrome c in treated cells, subcellular fractionation was performed before solubilization as previously described.20 Subsequently, apoptotic cytochrome c was measured in the cytosolic fraction according to the manufacturer's instructions. Cytochrome c levels were assayed by optical density measurement at 450 nm using an enzyme-linked immunosorbent assay microplate reader. Apoptotic cytochrome c levels were calculated from duplicate measurements and are expressed as percentage increase over control set as one.
Immunoblot analysis
Cells were solubilized at 4°C in lysis buffer consisting of 0.125 M Tris-Cl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 2.5% β-mercaptoethanol, and 10% glycerol. Protein concentration was determined by the Bio-Rad Dc protein assay and 50 to 100 μg of proteins were loaded onto a 12% SDS-polyacrylamide gel, subjected to electrophoresis, and transferred onto nitrocellulose membranes. After blocking of the membrane in 5% skimmed milk in Tris-buffered saline containing 0.05% Tween-20, the blots were incubated with specific antibodies. The blots were washed and protein bands were visualized using the ECL chemiluminescence system (GE Healthcare, Little Chalfont, United Kingdom).
Results
EAPB0203 induces growth arrest of malignant T cells
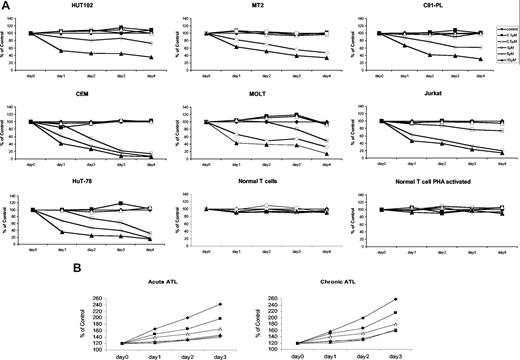
First, we determined whether EAPB0203 affects the growth of malignant T lymphocytes. We used 3 HTLV-I–transformed cell lines (HuT-102, MT-2, and C91-PL) and 4 HTLV-I–negative T-cell lines (CEM, Molt-4, Jurkat, and HuT-78). Cell growth was assessed by the CellTiter 96 cell proliferation assay kit. Achievable plasma concentrations of EAPB0203 ranging from 5 to 10 μM resulted in a dose- and time-dependent growth inhibition of all tested malignant T cells (Figure 2A), whereas lower doses, ranging from 0.1 to 0.5 μM, had no significant effect. Treatment at 1 μM had a moderate growth inhibitory effect on 3 HTLV-I–negative cell lines (CEM, Molt-4, and HuT-78), but not on Jurkat cells, or HTLV-I–infected cells. In general, HTLV-I–negative cells were more sensitive to EAPB0203 than HTLV-I–transformed cells (P < .01, Dunnett test). Significant growth inhibition was observed after 24 to 48 hours of treatment of HTLV-I–negative cell lines, at EAPB0203 concentrations of 5 to 10 μM, and complete growth inhibition (< 20% of control) was observed after 72 to 96 hours of treatment at these concentrations. In HTLV-I–infected cells, a significant but incomplete growth inhibition (> 30% of control) was observed even after treatment with 10 μM EAPB0203 for 96 hours. Importantly, normal resting or PHA-stimulated T cells from 2 healthy donors were completely resistant to EAPB0203 treatment up to 10 μM (Figure 2A and data not shown). By contrast, EAPB0203 almost completely inhibited the growth of freshly isolated ATL cells at concentrations of 1 to 10 μM (Figure 2B). Similar results were obtained using the trypan blue exclusion assay (data not shown).
EAPB0203 inhibited growth of HTLV-I–positive and –negative human T cell lines, and fresh ATL cells, but not normal resting and activated T lymphocytes. (A) Effects of EAPB0203 treatment on the growth of HTLV-I–positive (HuT-102, C91-PL, and MT-2) and HTLV-I–negative (CEM, Jurkat, Molt, and HuT-78) human T-cell lines, and normal resting or PHA-activated peripheral blood mononuclear cells (PBMCs). Activated normal PBMCs were supplemented with 2% PHA. EAPB0203 was added at the indicated concentrations in mol/L for 24 to 96 hours. Cells growth was assayed in triplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. The results are expressed as percentage of control (0.1% DMSO) and represent the means of the results obtained in at least 3 independent experiments. (B) Effects of EAPB0203 treatment on the growth of freshly isolated leukemic cells from one patient with acute ATL and one patient with chronic ATL. EAPB0203 was added at the indicated concentrations in mol/L for 24 to 72 hours. Cells growth was assayed in triplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. The results are expressed as percentage of day 0 control (0.1% DMSO) from one single experiment.
EAPB0203 inhibited growth of HTLV-I–positive and –negative human T cell lines, and fresh ATL cells, but not normal resting and activated T lymphocytes. (A) Effects of EAPB0203 treatment on the growth of HTLV-I–positive (HuT-102, C91-PL, and MT-2) and HTLV-I–negative (CEM, Jurkat, Molt, and HuT-78) human T-cell lines, and normal resting or PHA-activated peripheral blood mononuclear cells (PBMCs). Activated normal PBMCs were supplemented with 2% PHA. EAPB0203 was added at the indicated concentrations in mol/L for 24 to 96 hours. Cells growth was assayed in triplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. The results are expressed as percentage of control (0.1% DMSO) and represent the means of the results obtained in at least 3 independent experiments. (B) Effects of EAPB0203 treatment on the growth of freshly isolated leukemic cells from one patient with acute ATL and one patient with chronic ATL. EAPB0203 was added at the indicated concentrations in mol/L for 24 to 72 hours. Cells growth was assayed in triplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. The results are expressed as percentage of day 0 control (0.1% DMSO) from one single experiment.
EAPB0203 treatment causes G2/M cell-cycle arrest in malignant T cells
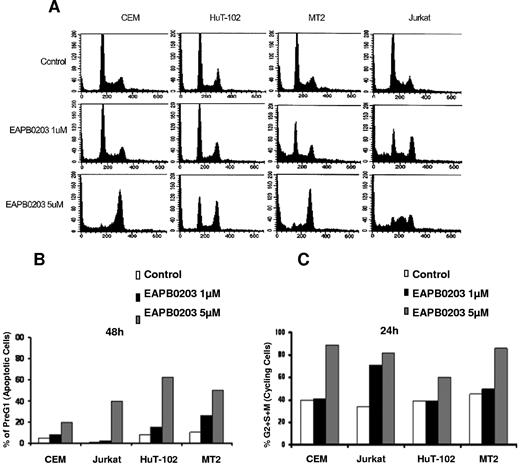
We then examined the cellular DNA contents distribution by FACS analysis on cell treatment. In all tested cell lines, EAPB0203 induced a significant dose-dependent increase in the pre-G0/G1 region, regarded as apoptotic cells (P < .01, Dunnett test). This increase was observed in both HTLV-I–negative and –positive cells (Figure 3A,B). EAPB0203 treatment also induced significant changes in the cell-cycle distribution (Figure 3). Figure 3C represents the percentage of cycling cells, the sum of (S + G2/M) phases, relative to nonapoptotic cells. Cycling CEM, Jurkat, HuT-102 and MT-2 cells significantly increased from 40% in the control cells to 60% to 85% of nonapoptotic cells after treatment with 5 μM EAPB0203 (P < .01). This increase was primarily the result of the increase in the percentage of cells arrested at the G2/M phases (Figure 3A-C). Similar results were observed with 1 μM EAPB0203in Jurkat cells (Figure 3A-C). These results indicate that, together with induction of apoptosis, EAPB0203 treatment induces a G2/M cell-cycle arrest in malignant T cells, which likely contributes to the growth inhibitory effects.
EAPB0203 induces G2/M cell-cycle arrest in HTLV-I–positive and –negative human T-cell lines. (A) Effects of EAPB0203 on the cell-cycle distribution of CEM, HuT-102, MT-2, and Jurkat. EAPB0203-treated cells were stained with PI (50 μg/mL), and the cell-cycle analysis was performed using a FACScan flow cytometer. (B) The pre-G1 percentage represents apoptotic cells. (C) Cycling cells, the sum of (S + G2/M) phases, are a percentage of nonapoptotic cells. The results are representative of 2 independent experiments.
EAPB0203 induces G2/M cell-cycle arrest in HTLV-I–positive and –negative human T-cell lines. (A) Effects of EAPB0203 on the cell-cycle distribution of CEM, HuT-102, MT-2, and Jurkat. EAPB0203-treated cells were stained with PI (50 μg/mL), and the cell-cycle analysis was performed using a FACScan flow cytometer. (B) The pre-G1 percentage represents apoptotic cells. (C) Cycling cells, the sum of (S + G2/M) phases, are a percentage of nonapoptotic cells. The results are representative of 2 independent experiments.
EAPB0203 treatment induced caspase-dependent apoptosis in malignant T cells
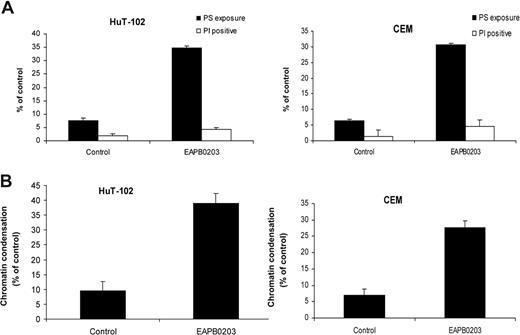
To confirm that the observed increase in the pre-G0/G1 results from apoptosis, EAPB0203-treated malignant T cells were analyzed using a double staining (4,6-diamidino-2-phenylindole, annexin V). Apoptotic cells exhibiting PS exposure were scored as annexin V+/PI−, whereas dead cells are PI+. Figure 4A shows a typical result obtained with HuT-102 and CEM cells. A significant increase in the apoptotic population was detected in both cell lines after treatment with 5 μM EAPB0203 for 24 hours. A similar experiment was also performed with Hoechst 33 342 staining (Figure 4B). This allows the evaluation of the percentage of chromatin condensation, which is a hallmark of apoptosis. Consistent with the above results, EAPB0203 treatment significantly increased the percentage of DNA degradation in both HuT-102 and CEM cells (Figure 4B and data not shown).
EAPB0203 induces apoptosis in HTLV-I–positive and –negative human T-cell lines. (A) Annexin-V binding. Cells were treated for 24 hours with EAPB0203 (5 μM). Using FITC-conjugated annexin-V membrane staining and PI nuclear counterstaining, apoptotic cells (FITC+ PI−) and dead cells either necrotic or postapoptotic (FITC+ PI+) were analyzed by fluorescence microscopy. (B) Hoechst 33 342 staining. Cells were treated with EAPB0203 (5 μM), stained with Hoechst 33 342, and analyzed under ultraviolet filter pack. The percentage of apoptotic cells displaying intensely fluorescent, condensed chromatin, are presented.
EAPB0203 induces apoptosis in HTLV-I–positive and –negative human T-cell lines. (A) Annexin-V binding. Cells were treated for 24 hours with EAPB0203 (5 μM). Using FITC-conjugated annexin-V membrane staining and PI nuclear counterstaining, apoptotic cells (FITC+ PI−) and dead cells either necrotic or postapoptotic (FITC+ PI+) were analyzed by fluorescence microscopy. (B) Hoechst 33 342 staining. Cells were treated with EAPB0203 (5 μM), stained with Hoechst 33 342, and analyzed under ultraviolet filter pack. The percentage of apoptotic cells displaying intensely fluorescent, condensed chromatin, are presented.
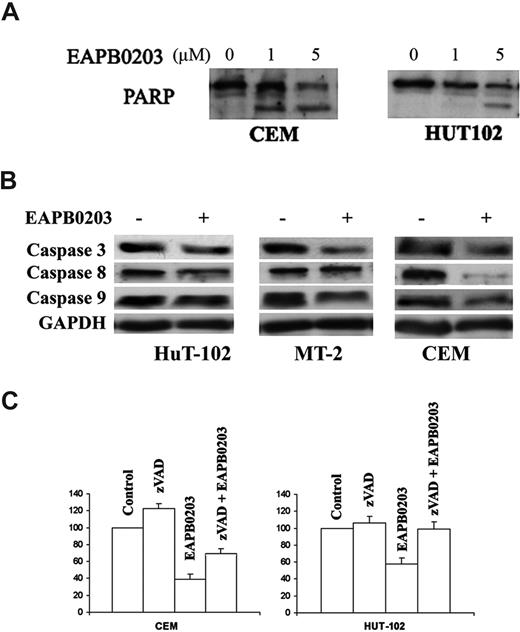
We then investigated whether the observed apoptosis was due or not to caspase activation. Cell extracts were obtained after various treatments and processed for Western blot. Indeed, in both CEM and HuT-102 cells, EAPB0203-induced apoptosis was associated with caspase activation, as shown by PARP cleavage (113 kDa) into its death-associated fragments (89 and 24 kDa). PARP cleavage was more pronounced in CEM cells and could be detected at 1 μM EAPB0203, whereas partial cleavage was observed in HuT-102 at 5 μM EAPB0203 only (Figure 5A). Results in Jurkat and MT-2 cells were similar to those obtained in CEM and HuT-102 cells, respectively (data not shown). Furthermore, EAPB0203 treatment resulted in cleavage of procaspase 3, procaspase 8, and procaspase 9 in all tested malignant T cells (Figure 5B and not shown). Ultimately, EAPB0203-induced growth inhibition of the HTLV-I–negative cells CEM and Jurkat cells, and of the HTLV-I–positive cells HuT-102 and MT-2, was partially but significantly reversed by the caspase inhibitor zVAD (P < .01) (Figure 5C and data not shown). Caspase inhibitors are known to inhibit cell-cycle progression in T and B lymphocytes.21,22 Hence, the protective effect of zVAD demonstrates the involvement of caspase activation and caspase-dependent apoptosis in EAPB0203-induced growth inhibition of HTLV-I–negative and –positive malignant T cells.
EAPB0203-induced apoptosis is caspase dependent in human T-cell lines. (A) Effect of EAPB0203 on the cleavage of PARP in HTLV-I–positive and –negative cells. Cells were treated for 24 hours with EAPB0203 at the indicated concentrations. Total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted against PARP. (B) Effect of EAPB0203 on the cleavage of pro-caspases 3, 8, and 9. (C) Effect of the general caspase inhibitor z-VAD (50 μM) on the growth inhibition induced by EAPB0203 in CEM and HuT-102 cells. Cell growth was assayed in triplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. Results are expressed as percentage of control plus or minus SD.
EAPB0203-induced apoptosis is caspase dependent in human T-cell lines. (A) Effect of EAPB0203 on the cleavage of PARP in HTLV-I–positive and –negative cells. Cells were treated for 24 hours with EAPB0203 at the indicated concentrations. Total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted against PARP. (B) Effect of EAPB0203 on the cleavage of pro-caspases 3, 8, and 9. (C) Effect of the general caspase inhibitor z-VAD (50 μM) on the growth inhibition induced by EAPB0203 in CEM and HuT-102 cells. Cell growth was assayed in triplicate wells with the CellTiter 96 nonradioactive cell proliferation kit. Results are expressed as percentage of control plus or minus SD.
EAPB0203-induced mitochondrial-mediated apoptosis
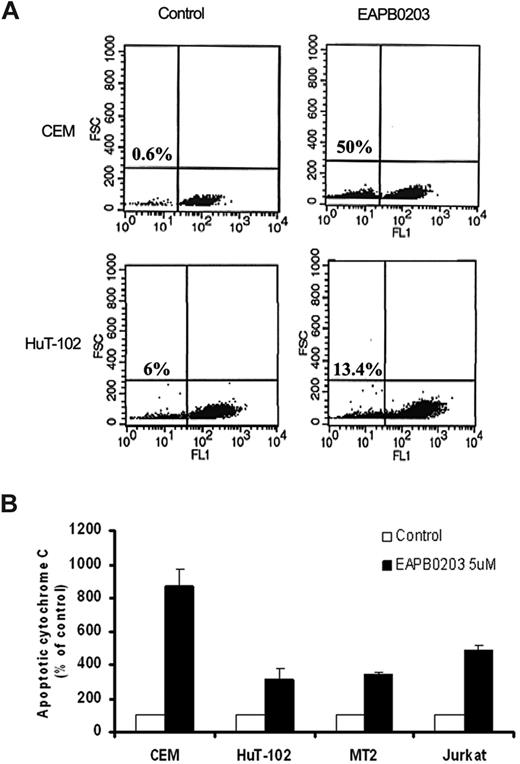
To assess for mitochondrial involvement in EAPB0203-induced apoptosis, ΔΨm was measured in HuT-102 and CEM cells (Figure 6A). Treatment with 5 μM EAPB0203 resulted in a dissipation of mitochondrial membrane potential as shown by the decrease in Rhodamine-123 fluorescence. This effect was evident in all tested ATL-derived and HTLV-I–negative cells (P < .01, Dunnett test).
Effect of EAPB0203 treatment on mitochondrial membrane potential and cytochrome c release. (A) CEM and HuT-102 cells were treated with EAPB0203 (5 μM) for 24 hours and stained with Rhodamine-123. Fluorescence emission at 525 nm after excitation at 488 nm was quantified by flow cytometer using CellQuest software. Histograms represent dot-plot analysis of Rhodamine-123 fluorescence (x-axis) over FSC (x-axis). Statistics representing the percentage of apoptotic cells with decreased mitochondrial membrane potential are displayed. (B) The HTLV-I–negative CEM and Jurkat, and the HTLV-I–positive HuT-102 and MT-2 malignant T cell lines were treated with EAPB0203 (5 μM) for 24 hours. Apoptotic cytochrome C was measured at the indicated time point in the cytosolic fractions as described in “Measurement of cytochrome c release.” Apoptotic cytochrome c levels were calculated from duplicate measurements and are expressed as percentage increase over control set as 100. Each bar represents the mean plus or minus range (n = 1). Results are representative of 2 independent experiments.
Effect of EAPB0203 treatment on mitochondrial membrane potential and cytochrome c release. (A) CEM and HuT-102 cells were treated with EAPB0203 (5 μM) for 24 hours and stained with Rhodamine-123. Fluorescence emission at 525 nm after excitation at 488 nm was quantified by flow cytometer using CellQuest software. Histograms represent dot-plot analysis of Rhodamine-123 fluorescence (x-axis) over FSC (x-axis). Statistics representing the percentage of apoptotic cells with decreased mitochondrial membrane potential are displayed. (B) The HTLV-I–negative CEM and Jurkat, and the HTLV-I–positive HuT-102 and MT-2 malignant T cell lines were treated with EAPB0203 (5 μM) for 24 hours. Apoptotic cytochrome C was measured at the indicated time point in the cytosolic fractions as described in “Measurement of cytochrome c release.” Apoptotic cytochrome c levels were calculated from duplicate measurements and are expressed as percentage increase over control set as 100. Each bar represents the mean plus or minus range (n = 1). Results are representative of 2 independent experiments.
The observed ΔΨm collapse secondary to altered mitochondrial membrane permeability suggests that caspase activation may result from the activation of the endogenous pathway of apoptosis, associated with cytochrome c release. Indeed, enzyme-linked immunosorbent assay assays demonstrated that EAPB0203 treatment resulted in a dramatic cytochrome c release in all tested malignant T cells (Figure 6B). This increase reached 300% of control levels in the HTLV-I–positive cells HuT-102 and MT-2, compared with 500% to 900% of control levels in the HTLV-I–negative cells CEM and Jurkat. These results indicate that EAPB0203-induced apoptosis of malignant T cells is associated with dissipation of mitochondrial membrane potential and cytochrome c release.
Effect of EAPB0203 treatment on antiapoptotic and cell-cycle regulatory proteins
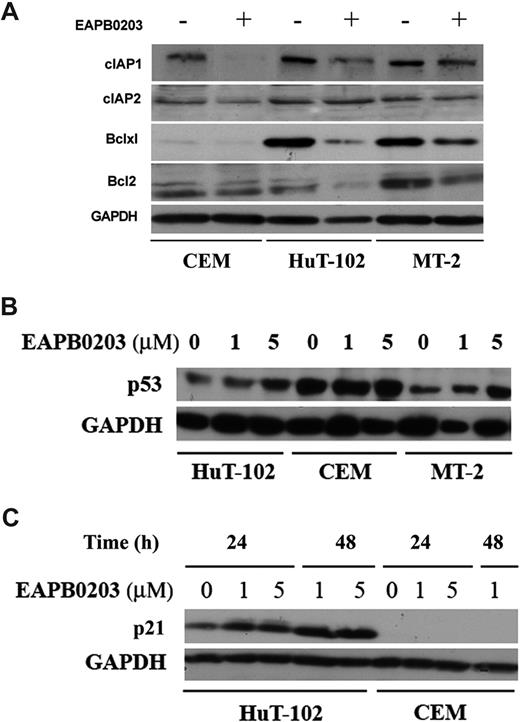
Activation of the mitochondrial-mediated, endogenous pathway of apoptosis is regulated by Bcl-2 family members. Tax has been reported to prevent apoptosis and caspase activation23 through up-regulation of the NF-κB–dependent XIAP (X-linked inhibitor of apoptosis), c-IAP-1 (inhibitor of apoptosis-1), and bcl-XL proteins. EAPB0203 treatment significantly down-regulated the protein levels of IAP-1 and bcl-XL, in both HTLV-I–positive and –negative malignant T cells (Figure 7A) and, to a lesser extent, c-IAP-2, XIAP, and bcl-2 proteins (Figure 7A). GAPDH Western blot (Figure 7A) confirmed equal loading of protein extracts. These results likely explain the EAPB0203-induced dissipation of mitochondrial membrane potential, cytochrome c release, and caspase-dependent apoptosis.
Effect of EAPB0203 treatment on antiapoptotic and cell-cycle regulatory proteins. (A) Effect of EAPB0203 treatment on the protein levels of the apoptosis regulatory proteins bcl-2, bcl-xl, or the caspase inhibitors c-IAP-1 and c-IAP-2. Cells were treated with EAPB0203 (5 μM) for 24 hours; total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted as described with specific antibodies. (B) Effect of EAPB0203 on the protein level of p53. Cells were treated with EAPB0203 at the indicated concentration for 24 hours; total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted with anti-p53 or GAPDH specific antibodies. (C) Effect of EAPB0203 on the protein level of p21. Cells were treated with EAPB0203 at the indicated concentration for 24 or 48 hours; total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted with anti-p21 or GAPDH specific antibodies.
Effect of EAPB0203 treatment on antiapoptotic and cell-cycle regulatory proteins. (A) Effect of EAPB0203 treatment on the protein levels of the apoptosis regulatory proteins bcl-2, bcl-xl, or the caspase inhibitors c-IAP-1 and c-IAP-2. Cells were treated with EAPB0203 (5 μM) for 24 hours; total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted as described with specific antibodies. (B) Effect of EAPB0203 on the protein level of p53. Cells were treated with EAPB0203 at the indicated concentration for 24 hours; total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted with anti-p53 or GAPDH specific antibodies. (C) Effect of EAPB0203 on the protein level of p21. Cells were treated with EAPB0203 at the indicated concentration for 24 or 48 hours; total SDS protein lysates (50-100 μg/lane) were prepared and immunoblotted with anti-p21 or GAPDH specific antibodies.
We then investigated the effect of EAPB0203 treatment on cell- cycle regulators. Treatment of ATL-derived cells HuT-102 and MT-2 with 5 μM EAPB0203 resulted in a significant increase in p21 and p53 protein levels (Figure 7B,C). In contrast, in the HTLV-I–negative cells CEM and Jurkat, EAPB0203 treatment had no effect on p53 protein, whereas p21 remained undetectable. Finally, gel shift assays showed that EAPB0203 treatment had no effect on the constitutive activation of the NF-κB pathway in HuT-102 and MT-2 cells (data not shown). These results suggest a potential involvement of the p53 pathway, in EAPB0203-induced growth inhibition of malignant T cells.
Discussion
We show that clinically achievable concentrations of EAPB0203, a member of the imidazo[1,2-a]quinoxalines, induce growth inhibition, G2/M cell-cycle arrest, and apoptosis in all tested malignant T cells, whereas no effect was observed on resting and activated normal T lymphocytes. The mechanism for this exquisite sensitivity of transformed cells to EAPB0203 remains to be elucidated.
The specific cellular target of EAPB0203 remains to be identified. Imiquimod binds to cell surface receptors, such as Toll-like receptors 7 and 8 (TLR-7 and TLR-8), thereby activating the MyD88-IRAK-TRAF6 pathway, subsequently leading to the activation of transcription factors, such as JNK, p38, AP1, and NF-κB.24,25 This eventually induces the secretion of pro-inflammatory cytokines. Imidazo[1,2-a]quinoxalines are imiquimod analogs that inhibit cyclic nucleotide phosphodiesterase enzymes 4, resulting in increased intracellular cAMP level and consequently cAMP response element binding protein phosphorylation.26 We recently showed that these imidazo[1,2-a]quinoxalines activate the p38 MAPK pathway and inhibit the PI3K pathway.11 The effective concentrations of imidazo[1,2-a]quinoxalines in T lymphocytes are compatible with the existence of imidazo[1,2-a]quinoxalines receptors on the cell surface. Hence, EAPB0203, a member of the imidazo[1,2-a]quinoxalines family, could also possibly bind TLR-7, TLR-8, or another member of the Toll receptor family.
In addition to indirect antitumor effects through binding to TLR-7 and TLR-8 on dendritic cells followed by secretion of a multitude of proinflammatory cytokines, recent data indicate that imiquimod also possesses direct proapoptotic activity against tumor cells both in vitro and in vivo. This novel mode of action involves caspase activation, presumably through Bcl2-dependent release of mitochondrial cytochrome c and subsequent activation of caspase 9, and consecutively caspase 3.10 Here we show that, in malignant T cells, EAPB0203 treatment significantly down-regulated the antiapoptotic proteins c-IAP-1 and Bcl-XL and resulted in a significant loss of mitochondrial membrane potential, cytoplasmic release of cytochrome c, and caspase activation. Importantly, EAPB0203-induced growth inhibition was reversed by caspase inhibitors, which inhibit cell-cycle progression in T and B lymphocytes.21,22 Hence, the protective effect of zVAD in EAPB0203-treated cells demonstrates the involvement of caspase activation, and caspase-dependent apoptosis, in EAPB0203-induced growth inhibition of malignant T cells. That HTLV-I–negative cells are more sensitive than their HTLV-I–positive counterpart to EAPB0203 effects is in agreement with previous reports that Tax protects HTLV-I–positive cells from caspase-dependent apoptosis23 through up-regulation of NF-κB–dependent apoptosis inhibitors, such as bcl-XL, or caspase inhibitors, such as c-IAP-1 and XIAP.
In addition to caspase activation, in HTLV-I–infected cells, EAPB0203 treatment resulted in a dramatic stabilization of p21 and p53 proteins in a dose- and time-dependent manner. In HTLV-infected cells, p53 is sometimes mutated but most frequently functionally inactive.27,28 Hence, EAPB0203-induced p53 may restore normal p53 function. In that sense, the up-regulation of p21, a well-characterized p53 target, argues in favor of this hypothesis. Interestingly, EAPB0203 effects on p21 and p53 proteins were limited to HTLV-I–positive cells, whereas no significant changes were seen in HTLV-I–negative malignant T cells. Similarly, treatment of HTLV-I–infected cells, but not HTLV-I-negative T cells, with the proteasome inhibitor PS-341 results in a dose- and time-dependent stabilization of p21 and p53 proteins,29 suggesting that EAPB0203 effects on p21 and p53 protein levels may occur at the posttranscriptional level through inhibition of their proteasomal degradation.
ATL remains of poor prognosis. Novel effective drugs are warranted to reduce the emergence of resistant clones. In addition, HTLV-I–negative peripheral T-cell lymphomas are also associated with poor prognosis because of a lower response rate to anthracyclin-based combination chemotherapy and a higher relapse rate compared with B-cell lymphomas.30 Our results support a potential therapeutic role for EAPB0203 in both ATL and HTLV-I–negative T-cell lymphomas, either as a systemic therapy or as topical treatment for lymphomatous skin lesions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the American University of Beirut Medical Practice Plan and University Research Board.
Authorship
Contribution: G.M. performed research and wrote the paper; H.E.-H. and Y.K. performed research; M.E.E.-S. designed research and wrote the paper; C.D.-M. designed and performed research, and wrote the paper; Y.L. performed research; O.H. designed research; P.-A.B. and A.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ali Bazarbachi, Department of Internal Medicine, American University of Beirut, PO Box 113-6044, Beirut, Lebanon; e-mail: bazarbac@aub.edu.lb.
References
Author notes
H.E.-H. and Y.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal