Abstract
The role of Gas6 in endothelial cell (EC) function remains incompletely characterized. Here we report that Gas6 amplifies EC activation in response to inflammatory stimuli in vitro. In vivo, Gas6 promotes and accelerates the sequestration of circulating platelets and leukocytes on activated endothelium as well as the formation and endothelial sequestration of circulating platelet-leukocyte conjugates. In addition, Gas6 promotes leukocyte extravasation, inflammation, and thrombosis in mouse models of inflammation (endotoxinemia, vasculitis, heart transplantation). Thus, Gas6 amplifies EC activation, thereby playing a key role in enhancing the interactions between ECs, platelets, and leukocytes during inflammation.
Introduction
The growth arrest-specific gene 6 (Gas6) binds to the receptor tyrosine kinases Axl, Tyro3, and Mer.1 Gas6 is composed of a N-terminal gamma-carboxy-glutamic acid domain (Gla-domain), a loop region, 4 EGF-like repeats, and a C-terminal steroid hormone binding globulin-like (SHBG-like) domain).1 Even though this molecule was discovered as a homologue of the anticoagulant protein S more than a decade ago, its role in vivo remains incompletely characterized.2,3 Originally identified in fibroblasts, Gas6 is expressed in various cell types, including endothelial cells (ECs),4 smooth muscle,5 and bone marrow cells.6 Gas6 and its receptors modify platelet activation and aggregation,7-10 but the role of Gas6 in the interplay between platelets and other cell types, such as ECs and leukocytes,11 during inflammatory conditions remains unclear
Several lines of evidence indeed suggest that Gas6 may affect ECs and leukocytes. ECs and leukocytes express Gas6 and its receptors, especially in conditions of inflammation and repair.4,12-16 Gas6 stimulates EC survival17-20 and promotes angiogenesis by enforcing the adhesion of Axl-expressing ECs via homophilic interactions,21-23 yet another study suggested that activation of Axl impairs tyrosine phosphorylation of vascular endothelial growth factor (VEGF) receptor-2.24 The activity of Gas6 on leukocytes also remains incompletely understood. Indeed, genetic loss of Mer inhibits cytokine production by natural killer cells25 while it stimulates tumor necrosis factor-α (TNF-α) production by monocytes14 and impairs clearance of apoptotic cells.26 Loss of all 3 Gas6 receptors, on the other hand, induces lymphoproliferative disorders via hyperactivation of antigen-presenting cells,27,28 but mice lacking Gas6 (Gas6−/−) do not develop autoimmune health problems (P.C., unpublished data, 2008). In humans, the plasma levels of Gas6 were found to be elevated during severe sepsis, a life-threatening condition involving increased interactions between ECs, leukocytes, and platelets.29,30 However, exogenous Gas6 inhibits granulocyte adhesion to ECs, but only at very high doses.31 Furthermore, the role of endogenous Gas6 in leukocyte extravasation in vivo was not studied. Here, by using our previously generated Gas6−/− mice,7 we studied whether Gas6 might play a role in EC activation and in the inter-actions between ECs, platelets, and leukocytes during inflammatory conditions.
Methods
Mice
The generation of Gas6−/− mice has been described.7 The original line was back-crossed into a C57BL/6 background for more than 10 generations, yielding congenic C57BL/6 Gas6−/− mice. Housing and all experimental animal procedures were approved by the Institutional Animal Care and Research Advisory Committee of the KU Leuven.
ELISA for mGas6
An enzyme-linked immunosorbent assay (ELISA) for murine Gas6 was developed using commercially available antibodies and standard (R&D Systems, Minneapolis, MN).
In vitro endothelial cell activation assays
Primary mouse ECs were isolated from matrigel granulomas.32 Production of Gas6 was determined after culturing 105 cells for 24 hours in low serum containing culture medium. Expression of adhesion molecules and cytokines was determined as described.33 In brief, ECs were plated at 2000 cells/well in 96-well tissue culture plates and grown at 37°C in 5% CO2 for 24 hours. At the time of the experiment, culture medium was removed and replaced by serum-free medium containing vehicle or recombinant mouse (rm)TNF-α (100 ng/mL; R&D Systems). After 24 hours, cells were washed once with serum-free medium. Specific antibodies for mICAM-1 and mVCAM-1 (R&D Systems) were added to each well and incubated at 37°C in 5% CO2 for 1 to 2 hours. After incubation with a horseradish peroxidase-conjugated secondary antibody, the horseradish peroxidase activity was determined. The data represent the mean plus or minus SEM of triplicates obtained from 3 different experiments. Levels of interleukin-6 (IL-6) and IL-1β in the culture medium were measured using commercially available ELISAs (R&D Systems). Human umbilical vein endothelial cells (HUVECs) were cultured in EGM medium (Cambrex, East Rutherford, NJ) supplemented with hEGF and hydrocortisone. Fresh Heparine and ECGS (HE) were added at each passage. For electroporation with Gas6, Axl, Tyro3, or control siRNA (QIAGEN, Basel, Switzerland), 90% confluent HUVECs were nucleofected using the Nucleofector device (Amaxa, Gaithersburg, MD) according to the manufacturer's instruction (nucleofector program A-034). At 24 hours after nucleofection, HUVECs were stimulated for 24 hours with rhTNF-α (R&D Systems) in medium without HE. Cell lysates were analyzed for hICAM-1 expression using a commercially available ELISA (R&D Systems).
In vivo study of platelet tethering, rolling, and adhesion
Videomicroscopy on mesenteric venules was performed as described with slight modifications.34 Briefly, recipient C57Bl6 WT or Gas6−/− mice were anesthetized with urethane (1.4 mg/kg) to maintain physiologic blood pressure and heart rate, and the external jugular vein was cannulated for continuous venous access. Nonactivated platelets of donor syngeneic WT or Gas6−/− mice (1 donor per 1 recipient mouse) were fluorescent-labeled with calcein and slowly infused via the catheter. Alternatively, rhodamine 6G (1 mg/mL in saline) was infused to label circulating cells in vivo; platelets and leukocytes were clearly distinguishable by size. Gallic acid (GA; 3.4 mg/kg per hour), previously shown to selectively inhibit P-selectin, was continuously administered intravenously via an infusion pump, as described previously.34,35 The mesentery of recipient mice was externalized, and mesenteric venules were exposed on the table of an epifluorescence microscope for direct visualization under videomicroscopy. Local EC activation was induced via topical application of the calcium ionophore A23187 (10 μL at 30 μM). Platelet activation was induced by intravenous bolus injection of collagen (50 μg/kg), combined with a αIIbβ3 antagonist (G4120, 1 mg/kg) to prevent platelet aggregation. Movies were recorded 10 minutes after activation at 10 frames per second and analyzed post-hoc using NIH Image v1.62 software. For kinetic analysis, movies with a duration of 1 minute were recorded at indicated time points. The following criteria were used: cells were tethering/rolling when a clear cell/stripe was present on 3 or more consecutive frames; cells were adhering/arrested when a clear arrest of cells was present on 2 or more consecutive frames; conjugates were interacting when a clearly more intense fluorescence signal containing at least 2 separately identifiable platelets was present on 3 or more consecutive frames. Data were expressed as cells per minute. The mean number of infused platelets (± 50 × 106 in 200 μL) and the sizes of the analyzed mesenteric venules (120-150 μm diameter) were not different between genotypes. Determination of Weibel-Palade body count and fluorescent-activated cell sorter analysis are described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
In vivo model of endothelial cell activation
Mice were injected intraperitoneally with a sublethal dose of recombinant mouse TNF-α (100 μg/kg; R&D Systems), hearts were harvested after 24 hours and immediately embedded in Tissue-Tek in liquid nitrogen-cooled 2-methylbutane; 7 μm sagittal sections were briefly fixed in acetone and immunostained with a biotinylated rat anti–VCAM-1 (1:50, BD Biosciences, San Jose, CA) or a rat anti–ICAM-1 antibody (CD54, 1:100, Seikagaku America, Rockville, MD). Tissues were stained by amplification with the tyramide signal amplification system (PerkinElmer Life and Analytical Sciences, Waltham, MA) using 3,3′-diaminobenzidine (Sigma-Aldrich, St Louis, MO) as a chromogen substrate and counterstained with Harris hematoxylin.
Animal models
All animal models are described in Document S1.
Statistical analysis
SPSS 11.0 was used for statistical analysis, and P values were calculated using unpaired Student t test, ANOVA, or Fisher exact analyses. Data are represented as mean plus or minus SEM and were considered statistically significant at P less than .05.
Results
Endothelial cells release Gas6
Gas6 is present in mouse blood plasma36 ; recent reports suggest that ECs, which express Gas6,4 might be a potential source of plasma Gas6.29,37 By ELISA, Gas6 was indeed detectable in plasma of wild-type (WT) mice (29 ± 2 ng/mL) but not in Gas6−/− mice. Gas6 was also detectable in murine platelets (28 ± 3 pg/106 platelets) but undetectable in erythrocyte lysates. In baseline conditions (ie, low serum without supplementation of any inflammatory cytokine), cultured murine primary ECs constitutively released Gas6 in the conditioned medium (708 ± 50 pg/106 cells per 24 hours; N = 5).
Endothelial Gas6 promotes activation of endothelial cells
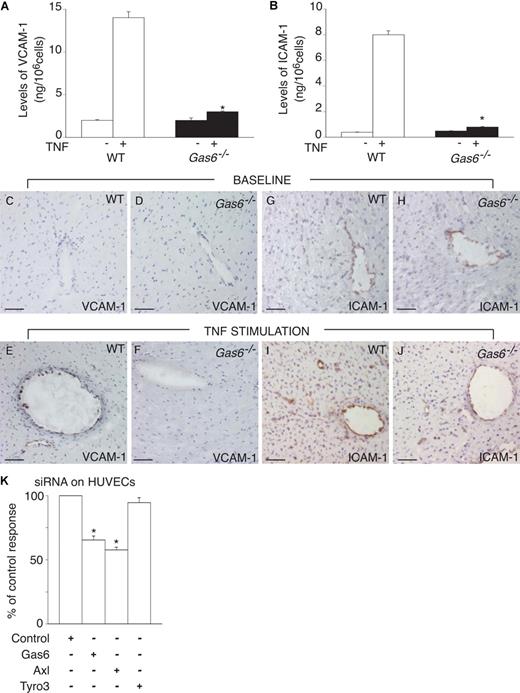
To study the role of endothelial Gas6, we first investigated the response of cultured ECs, derived from WT and Gas6−/− mice, to TNF-α. After stimulating ECs for 24 hours with 100 ng/mL TNF-α, we analyzed the endothelial expression of the activation markers VCAM-1 and ICAM-1, and the release of the inflammatory cytokines IL-1β and IL-6. In baseline conditions (ie, without TNF-α), we found no genotypic differences in the expression of the adhesion molecules (Figure 1A,B) or in the release of cytokines (ng per 106 cells per 24 hours: 0.6 ± 0.1 for IL-1β and 1 ± 0.1 for IL-6 in WT cells vs 0.5 ± 0.03 for IL-1β and 0.8 ± 0.05 for IL-6 in Gas6−/− cells; P = not significant). On TNF-α stimulation, WT ECs expressed elevated levels of VCAM-1 and ICAM-1 (Figure 1A,B), and released approximately 10-fold more IL-1β and IL-6 (ng per 106 cells per 24 hours: 6 ± 1 for IL-1β and 11 ± 1 for IL-6; P < .05 vs control). In contrast, in the absence of Gas6, TNF-α failed to activate ECs as evidenced by the lower levels of adhesion molecules (Figure 1A,B) and cytokine release (ng per 106 cells per 24 hours: 0.9 ± 0.1 for IL-1β and 2 ± 1 for IL-6; P = not significant vs control; P < .05 vs WT). The defective up-regulation of VCAM-1 and ICAM-1 expression in Gas6−/− ECs was confirmed in vivo. Indeed, by immunostaining, fewer ECs in the heart expressed VCAM-1 and ICAM-1 at 24 hours after a systemic challenge with TNF-α in Gas6−/− than WT mice (Figure 1C-J). The increased response of WT ECs to TNFα was, however, not the result of an increased release of Gas6 by ECs, stimulated with TNF-α (pg/106 cells/24 hours: 708 ± 50 in baseline vs 669 ± 58 after TNFα; N = 5; P = not significant).
Gas6 regulates EC activation in vitro and in vivo. (A,B) Stimulation of ECs with 100 ng/mL TNF-α revealed a role for Gas6 in the activation of endothelial cells in vitro. TNF-α stimulated the production of VCAM-1 (A) and ICAM-1 (B) by WT ECs but not by Gas6−/− ECs. Data represent means plus or minus SEM (*P < .05 vs stimulated WT cells, N = 9). (C-J) In normal heart tissue (baseline), no (VCAM-1) or limited (ICAM-1) expression of the adhesion molecules VCAM-1 (C,D) and ICAM-1 (G,H) can be detected in the blood vessels of either WT or Gas6−/− mice. At 24 hours after intraperitoneal injection of a sublethal dose of TNF-α, up-regulated expression of VCAM-1 (E) and ICAM-1 (I) can be seen in the endothelial cells in WT hearts but is absent (VCAM-1) or reduced (ICAM-1) in Gas6−/− hearts (F-J). Bar represents 50 μm in all panels. (K) Knockdown of Gas6 or Axl in HUVECs by transfection of cells with 25 nM siRNA for, respectively, Gas6 or Axl, reduced the expression of hICAM-1 in response to TNF-α (10 ng/mL). In contrast, knockdown of Tyro3, using 100 nM of Tyro3 siRNA, failed to reduce the expression of hICAM-1 on HUVECs in response to TNF-α (10 ng/mL). Data represent the percentage of the control response (*P < .05 vs control siRNA, N = 3).
Gas6 regulates EC activation in vitro and in vivo. (A,B) Stimulation of ECs with 100 ng/mL TNF-α revealed a role for Gas6 in the activation of endothelial cells in vitro. TNF-α stimulated the production of VCAM-1 (A) and ICAM-1 (B) by WT ECs but not by Gas6−/− ECs. Data represent means plus or minus SEM (*P < .05 vs stimulated WT cells, N = 9). (C-J) In normal heart tissue (baseline), no (VCAM-1) or limited (ICAM-1) expression of the adhesion molecules VCAM-1 (C,D) and ICAM-1 (G,H) can be detected in the blood vessels of either WT or Gas6−/− mice. At 24 hours after intraperitoneal injection of a sublethal dose of TNF-α, up-regulated expression of VCAM-1 (E) and ICAM-1 (I) can be seen in the endothelial cells in WT hearts but is absent (VCAM-1) or reduced (ICAM-1) in Gas6−/− hearts (F-J). Bar represents 50 μm in all panels. (K) Knockdown of Gas6 or Axl in HUVECs by transfection of cells with 25 nM siRNA for, respectively, Gas6 or Axl, reduced the expression of hICAM-1 in response to TNF-α (10 ng/mL). In contrast, knockdown of Tyro3, using 100 nM of Tyro3 siRNA, failed to reduce the expression of hICAM-1 on HUVECs in response to TNF-α (10 ng/mL). Data represent the percentage of the control response (*P < .05 vs control siRNA, N = 3).
The effect of Gas6 was not restricted to murine ECs, but Gas6 also regulated the activation of human ECs. Indeed, Gas6 was expressed in HUVECs (mRNA levels normalized for transcript levels of TATA box binding protein [TBP], 26.1 ± 0.3; N = 3). Knockdown with a Gas6-selective RNAi construct reduced the Gas6 expression in HUVECs by −75% and reduced the expression of ICAM-1 in response to TNF-α, whereas a control plasmid (encoding a mismatched sequence) was ineffective (Figure 1K). Thus, Gas6 promotes the responsiveness of ECs to an inflammatory stimulus.
Axl mediates the endothelial response to Gas6
We also assessed which of the Gas6 receptors (Tyro-3, Axl, or Mer) on ECs mediated the response to Gas6. All 3 receptors were expressed in HUVECs (mRNA expression levels normalized to those of the TBP: 135 ± 3 for Axl, 13.4 ± 1.0 for Mer, and 3.2 ± 0.3 for Tyro3; N = 3). Transfection of HUVECs with siRNAs for Axl or Tyro3 efficiently reduced the expression of Axl and Tyro3 by approximately 70% and approximately 80%, respectively (mRNA levels normalized to those of TBP: 45 ± 3 for Axl and 0.5 ± 0.1 for Tyro3; N = 3). Knockdown of these Gas6 receptors did not affect EC expression levels of ICAM-1 in baseline conditions. However, the up-regulation of ICAM-1 in response to TNF-α was reduced on knockdown of Axl, but not of Tyro3 (Figure 1K). Unfortunately, knockdown of Mer failed to reduce the expression of Mer in HUVECs (mRNA levels normalized to those of the TBP: 11.6 ± 1.4; N = 3), precluding further analysis. Thus, binding of Gas6 to Axl is dispensable for baseline expression of ICAM-1 but necessary for the TNF-α-induced expression of ICAM-1.
Gas6 promotes the sequestration of platelets onto endothelium
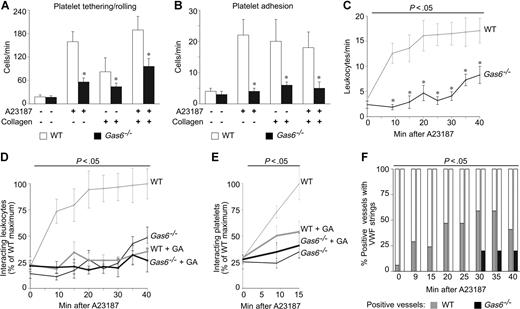
Prompted by the finding that loss or knock-down of Gas6 in ECs impaired their activation in response to inflammatory stimuli in vitro, we investigated the role of Gas6 in EC activation in vivo. One of the predominant features of inflamed endothelium is the enhanced interactions with circulating cells such as platelets and leukocytes, leading to increased tethering, rolling, firm adhesion, extravasation and tissue infiltration of leukocytes.11,38 To study the role of Gas6, we first determined, by intravital videomicroscopy, the tethering, rolling and adhesion of calcein-labeled platelets onto mesenteric venules in WT and Gas6−/− mice.34,39 In baseline conditions, only a small number of platelets sequestered onto ECs in either genotype (Figure 2A-B). To selectively activate the endothelium, venules were locally superfused with the calcium ionophore A23187. Significantly more platelets interacted with the activated endothelium in WT than Gas6−/− mice (Figure 2A,B), indicating that ECs, activated in the absence of Gas6, failed to efficiently sequester platelets.
Gas6 promotes endothelial sequestration of platelets and leukocytes via P-selectin. (A,B) In vivo videomicroscopy of mesenteric venules revealed impaired platelet-EC interactions in Gas6−/− mice. Compared with WT mice, fewer platelets tethered/rolled (A) and adhered (B) onto the endothelial surface of the venules after single or combined activation of ECs and platelets. The endothelium was selectively activated by topical administration of A23187. Platelets were selectively activated by injection of collagen together with a GPIIb/IIIa antagonist to avoid aggregation (*P < .05 vs WT, N = 10–21). (C) Loss of Gas6 delayed and impaired the sequestration of leukocytes onto the endothelium, activated by topical administration of A23187. P less than .05 versus WT (ANOVA; *P < .05 vs WT per time point, N = 10-16). (D,E) Impaired and delayed sequestration of leukocytes (D) and platelets (E) onto activated endothelium in Gas6−/− mice can be explained by reduced P-selectin expression as P-selectin inhibition, by systemic infusion of GA, effectively reduced the interactions between circulating cells and the activated endothelium in WT mice but not in Gas6−/− mice. Data were expressed as percentage of the maximal value in WT mice. P less than .05 versus WT (ANOVA; N = 7-16 for leukocytes; N = 7-10 for platelets). (F) Release of VWF strings was impaired and delayed in Gas6−/− mice after topical administration of A23187. Data were expressed as percentage of positive vessels per time point (P < .05 vs WT, ANOVA; N = 10-16).
Gas6 promotes endothelial sequestration of platelets and leukocytes via P-selectin. (A,B) In vivo videomicroscopy of mesenteric venules revealed impaired platelet-EC interactions in Gas6−/− mice. Compared with WT mice, fewer platelets tethered/rolled (A) and adhered (B) onto the endothelial surface of the venules after single or combined activation of ECs and platelets. The endothelium was selectively activated by topical administration of A23187. Platelets were selectively activated by injection of collagen together with a GPIIb/IIIa antagonist to avoid aggregation (*P < .05 vs WT, N = 10–21). (C) Loss of Gas6 delayed and impaired the sequestration of leukocytes onto the endothelium, activated by topical administration of A23187. P less than .05 versus WT (ANOVA; *P < .05 vs WT per time point, N = 10-16). (D,E) Impaired and delayed sequestration of leukocytes (D) and platelets (E) onto activated endothelium in Gas6−/− mice can be explained by reduced P-selectin expression as P-selectin inhibition, by systemic infusion of GA, effectively reduced the interactions between circulating cells and the activated endothelium in WT mice but not in Gas6−/− mice. Data were expressed as percentage of the maximal value in WT mice. P less than .05 versus WT (ANOVA; N = 7-16 for leukocytes; N = 7-10 for platelets). (F) Release of VWF strings was impaired and delayed in Gas6−/− mice after topical administration of A23187. Data were expressed as percentage of positive vessels per time point (P < .05 vs WT, ANOVA; N = 10-16).
We previously showed that Gas6−/− platelets exhibited impaired activation and aggregation7,10 but did not investigate whether they also interact less with the endothelial surface. When selectively activating the platelets (by injecting collagen together with an αIIbβ3 antagonist to avoid aggregation), tethering, rolling, and adhesion of platelets on ECs in vivo were impaired in the absence of Gas6 (Figure 2A,B). Importantly, endothelial sequestration of single platelets was also impaired in Gas6−/− mice when both platelets and endothelium were activated (Figure 2A,B). Thus, Gas6 promotes the sequestration of circulating platelets onto activated ECs in vivo.
Gas6 promotes leukocyte sequestration on endothelium
To study the role of Gas6 in the sequestration of leukocytes on ECs, we also determined, by intravital videomicroscopy, the interactions of circulating leukocytes with the mesenteric endothelium, using another labeling protocol (in vivo labeling via rhodamine-G infusion40,41 ). Because leukocytes progressively sequester onto the activated endothelium,34 we analyzed both the number of sequestered leukocytes and the kinetics of their sequestration in response to A23187. In baseline conditions, only a small number of leukocytes interacted with the mesenteric endothelium in either genotype (Figure 2C). When the endothelium was selectively activated by topical A23187 administration, leukocyte sequestration progressively increased in WT mice, reaching a plateau phase at 20 minutes after activation (Figure 2C). In contrast, in Gas6−/− mice, leukocyte sequestration was not only delayed but, in addition, significantly fewer leukocytes interacted with the activated endothelium at all time points in the absence of Gas6 (Figure 2C). Taken together, Gas6 amplifies and accelerates the sequestration of circulating leukocytes onto activated ECs.
P-selectin, a downstream mediator of Gas6?
P-selectin promotes the rapid sequestration of leukocytes and platelets on ECs, early after their activation.34,38,41-45 We therefore reasoned that the defective sequestration of circulating cells on activated ECs in Gas6−/− mice might be attributable to an impaired up-regulation of P-selectin on these cells. In view of the lack of high-quality antibodies to reliably and reproducibly detect P-selectin expression on endothelium, we resorted to a functional characterization of the role of P-selectin in vivo. If the activity of Gas6 would rely, at least in part, on up-regulation of P-selectin, then P-selectin inhibitors would be expected to reduce the sequestration of circulating cells on ECs in WT but not in Gas6−/− mice. To neutralize P-selectin in vivo, we used GA, a well-known polyphenol acting as a selective antagonist of P-selectin, but not of E-selectin.34,35,46 As expected (because P-selectin is poorly expressed on quiescent ECs), GA did not alter the interactions of leukocytes with resting endothelium in either genotype (Figure 2D). However, when the endothelium was selectively activated by topical administration of A23187, GA inhibited the interactions of leukocytes with ECs in WT mice, notably, to the same level as in Gas6−/− mice, whereas GA was ineffective in Gas6−/− mice (Figure 2D). Similar effects were observed when studying the effect of GA on the sequestration of platelets on ECs (Figure 2E). Thus, P-selectin is a likely downstream mediator of Gas6 in promoting the sequestration of leukocytes and platelets on activated ECs.
To further underscore that Gas6 indeed induced the surface expression of P-selectin, we analyzed whether Gas6 affected the secretion of other factors, known to be coreleased with P-selectin. The hemostatic protein von Willebrand factor (VWF) is such a molecule, stored together with P-selectin in endothelial Weibel-Palade bodies and coreleased from these stores on EC activation.38,47,48 Videomicroscopy revealed that EC activation by A23187 in WT mice exposed VWF on the luminal surface of ECs, visualized as “platelet strings” sequestered onto VWF multimers.39 In contrast, in Gas6−/− mice, fewer platelet strings were detected and only at later time points (Figure 2F). The reduced VWF-platelet strings in Gas6−/− mice were not attributable to an impaired capacity of Gas6−/− platelets to bind VWF (not shown) or to a reduced number of Weibel-Palade bodies in Gas6−/− ECs (total volume of Weibel-Palade bodies per image stack in computed voxels: 27 400 ± 7030 in WT ECs vs 32 100 ± 8250 in Gas6−/− ECs; N = 8; P = not significant; Figure S1). Together, these findings suggest that, in the absence of Gas6, endothelial degranulation upon EC activation with A23187 is impaired.
Gas6 promotes the sequestration of platelet-leukocyte conjugates
Recent reports indicate that platelets play a pivotal role in the sequestration of leukocytes on ECs in a P-selectin–dependent manner.11,45 Indeed, through P-selectin, platelets bind to the P-selectin ligand PSGL on leukocytes, and thereby form multicellular conjugates, which allows them to release cytokines to further activate the leukocytes.11,49 These conjugates of P-selectin+ platelets / PSGL+ leukocytes tether and roll on ECs, with a higher avidity than unconjugated leukocytes, thereby enhancing endothelial inflammation.42,44 In addition, the P-selectin–mediated leukocyte activation, which results from leukocyte conjugation with platelets, further promotes leukocyte sequestration to the endothelium and subsequent extravasation, overall leading to an amplified inflammatory response.41,43,46 We previously showed that P-selectin levels on activated platelets were reduced in the absence of Gas6.7 Here, we analyzed, by flow cytometry, the formation of heteroconjugates, consisting of CD41/61+ platelets and CD45+ granulocytes in WT and Gas6−/− mice. Comparable amounts of conjugates were present in baseline conditions in both genotypes (percentage of total number of granulocytes: 1.2 ± 0.2% in Gas6−/− mice vs 1.3 ± 0.4% in WT mice; N = 5; P = not significant). However, after administration of lipopolysaccharide (LPS) (40 mg/kg intraperitoneally), fewer conjugates formed in Gas6−/− than WT animals (3.0 ± 0.4% in Gas6−/− mice vs 4.7 ± 0.6% in WT mice; N = 5; P < .05). A similar 4-fold increase in heteroconjugate formation was recently shown to substantially activate circulating monocytes.46
By videomicroscopy, we also found that loss of Gas6 impaired the formation and the tethering/rolling of these conjugates onto the endothelium. In baseline conditions, no genotypic differences were found in the number of conjugates visualized per minute in the mouse mesenteric circulation (3 ± 1 in WT mice vs 2 ± 1 in Gas6−/− mice; N = 10–16; P = not significant), and none of them tethered/rolled. On selective activation of platelets (“Gas6 promotes the sequestration of platelets onto endothelium”), fewer conjugates were visualized in Gas6−/− mice (conjugates observed per minute: 32 ± 9 in WT mice vs 5 ± 2 in Gas6−/− mice; N = 10-14; P < .05). Correspondingly, conjugates interacted more with the endothelium in WT than Gas6−/− mice (conjugates tethering/rolling per minute: 5 ± 2 in WT mice vs 2 ± 1 in Gas6−/− mice; N = 10-14; P < .05). After activation of both platelets and ECs, fewer conjugates formed and tethered/rolled onto the endothelium in Gas6−/− mice (conjugates observed per minute: 36 ± 6 in WT mice vs 4 ± 1 in Gas6−/− mice; conjugates tethering/rolling per minute: 17 ± 5 in WT mice vs 1 ± 1 in Gas6−/− mice; N = 12-15; P < .05). Thus, Gas6 promotes both the formation and endothelial sequestration of platelet-leukocyte conjugates, likely by regulating the expression levels of platelet and endothelial P-selectin. Overall, Gas6 participates in the inflammatory response between ECs, platelets, and leukocytes, by regulating the release of tethering and adhesion receptors in these cells.
Gas6 promotes leukocyte extravasation during inflammation
The above-mentioned findings suggested that Gas6 might play a role in augmenting the inflammatory response in vivo. To further underscore this hypothesis experimentally, we exposed Gas6−/− mice to a panel of inflammatory challenges in vivo, including endotoxinemia, vasculitis, and heterotopic heart transplantation. To characterize leukocyte infiltration in inflamed tissue, we systemically injected LPS, a model of endotoxinemia, in WT and Gas6−/− mice. As expected, within 4 hours after intraperitoneal injection of 20 mg/kg LPS, pulmonary myeloperoxidase activity levels, a measure of infiltrated leukocytes, increased in WT mice (absorbance units/mg tissue: 11 ± 2 after saline vs 23 ± 3 after LPS; N = 7-12; P < .05). In contrast, in Gas6−/− mice, pulmonary myeloperoxidase levels failed to increase at all (absorbance units/mg tissue: 8 ± 3 after saline vs 10 ± 2 after LPS; N = 5-8; P = not significant), suggesting that extravasation of inflammatory cells was impaired in Gas6−/− mice. CD45 immunostaining confirmed that fewer CD45+ leukocytes extravasated in the lungs of Gas6−/− than WT animals (Figure 3A,B). Thus, Gas6 is required for leukocyte extravasation in conditions of systemic inflammation.
In vivo role of Gas6 in inflammation and thrombosis. (A,B) CD45 staining in lungs of WT and Gas6−/− mice 4 hours after intraperitoneal injection of 20 mg/kg LPS showed higher neutrophil extravasation in the lung parenchyme of WT mice (A) compared with Gas6−/− animals (B). (C-D) CD45 immunostaining in footpads of WT and Gas6−/− animals, injected with 50 μg LPS, revealed extensive inflammation in the adventitial and muscle area of WT animals (E), whereas vessels in Gas6−/− animals were less inflamed (D). (E,F) Hematoxylin and eosin staining in footpads of WT and Gas6−/− animals, injected with 50 μg LPS, revealed the presence of large, organized thrombi covering most of the lumen in veins of WT animals (E), whereas vessels in Gas6−/− animals contained fewer and smaller thrombi (F). Magnification bar represents 50 μm in all panels.
In vivo role of Gas6 in inflammation and thrombosis. (A,B) CD45 staining in lungs of WT and Gas6−/− mice 4 hours after intraperitoneal injection of 20 mg/kg LPS showed higher neutrophil extravasation in the lung parenchyme of WT mice (A) compared with Gas6−/− animals (B). (C-D) CD45 immunostaining in footpads of WT and Gas6−/− animals, injected with 50 μg LPS, revealed extensive inflammation in the adventitial and muscle area of WT animals (E), whereas vessels in Gas6−/− animals were less inflamed (D). (E,F) Hematoxylin and eosin staining in footpads of WT and Gas6−/− animals, injected with 50 μg LPS, revealed the presence of large, organized thrombi covering most of the lumen in veins of WT animals (E), whereas vessels in Gas6−/− animals contained fewer and smaller thrombi (F). Magnification bar represents 50 μm in all panels.
Gas6 promotes vasculitis
We also injected LPS in the footpad of mice to induce localized vasculitis and thrombosis (“Schwartzman reaction”). Injection of 50 μg LPS (in 20 μL saline) in the footpad caused redness, swelling, and temperature elevation of the footpad in both genotypes. However, semiquantitative scoring of redness and swelling revealed that, compared with WT mice, these symptoms were less severe in Gas6−/− mice. Indeed, when scoring the redness on a scale from 0 to 3 (ranging from “no redness” to “light red” over “dark red” to “gangrene”), a significant genotypic difference was found after injection 50 μg LPS (redness score: 2.4 ± 0.2 in WT mice vs 1.6 ± 0.2 in Gas6−/− animals; N = 6; P < .05). Histologic analysis showed that fewer leukocytes accumulated in the adventitia of vessels in Gas6−/− mice (CD45+ cells, percentage of all cells per microscopic field: 68 ± 5% in WT mice vs 10 ± 4% in Gas6−/− mice; N = 6; P < .05, Figure 3C,D), suggesting that loss of Gas6 attenuated the severity of vasculitis. In addition, fewer leukocytes infiltrated the inflamed muscle in Gas6−/− mice (CD45+ cells, percentage of all cells per microscopic field: 51 ± 12% in WT mice vs 29 ± 15% in Gas6−/− mice; N = 6; P < .05, Figure 3C,D). Hence, loss of Gas6 impaired leukocyte extravasation in conditions of local inflammation.
Venous thrombosis during vasculitis was also reduced in Gas6−/− mice. Indeed, injection of 50 μg LPS caused venous thrombosis in 88% of veins in WT mice, but only in 44% of veins in Gas6−/− mice (P < .05; Figure 3E,F). Arteries characteristically contained fewer thrombi; injection of 50 μg LPS caused arterial thrombosis in 10% of arteries in WT mice but in none of the arteries in Gas6−/− mice. Hence, the impaired vascular inflammation in Gas6−/− mice was accompanied by a reduced incidence of venous thrombosis.
Gas6 accelerates graft destruction of transplanted hearts
In an established mouse model of heterotopic heart transplantation,50 acute graft destruction, as a consequence of an extended warm transplant ischemia time, is primarily triggered by an intense inflammatory cell infiltration.51 WT or Gas6−/− mouse donor hearts were grafted in syngeneic WT or Gas6−/− recipients, respectively, and beating of the transplanted hearts was followed daily to determine graft destruction. On grafting of WT hearts into WT recipients, 10 of the 11 grafts stopped beating within 7 to 12 days because of profound inflammatory cell infiltration, and myocarditis, resulting in cardiomyocyte necrosis (Figure 4A,C,E). VWF immunostaining in combination with a nuclear counterstaining revealed that platelets, leukocytes, and platelet/leukocyte conjugates often adhered to the wall of the coronary vessels in the graft (Figure 4G,H).
Role of Gas6 in graft destruction of mouse heart grafts exposed to warm ischemia. (A-F) Hematoxylin and eosin (A,D) and CD45 (E,F) stainings of sections through WT and Gas6−/− mouse heart grafts, 10 days after transplantation, revealed profound inflammatory cell infiltration and myocardial cell necrosis (A,C,E) in the WT heart graft. In contrast, the Gas6−/− transplanted heart graft showed a remarkably normal morphology and only limited leukocyte infiltration (B,D,F). (G-I) Immunostaining for VWF (brown) combined with nuclear counterstaining with Harris hematoxylin (blue) revealed platelets (arrowhead in panel H), leukocytes, and platelet-leukocyte conjugates (arrowhead in panel G) adhering to the wall of the coronary vessels in the graft of WT mice, whereas this was not seen in hearts transplanted in Gas6−/− mice (I). VCAM-1 (J,K) and ICAM-1 (L,M) up-regulated expression is reduced in Gas6−/− hearts transplanted into Gas6−/− mice (K,M) compared with WT cardiac grafts, dissected 3 days after transplantation into WT mice (J,L). Bar represents 100 μm in panels A and B, 50 μm in panels C-F and J-M, and 10 μm in panels G-I.
Role of Gas6 in graft destruction of mouse heart grafts exposed to warm ischemia. (A-F) Hematoxylin and eosin (A,D) and CD45 (E,F) stainings of sections through WT and Gas6−/− mouse heart grafts, 10 days after transplantation, revealed profound inflammatory cell infiltration and myocardial cell necrosis (A,C,E) in the WT heart graft. In contrast, the Gas6−/− transplanted heart graft showed a remarkably normal morphology and only limited leukocyte infiltration (B,D,F). (G-I) Immunostaining for VWF (brown) combined with nuclear counterstaining with Harris hematoxylin (blue) revealed platelets (arrowhead in panel H), leukocytes, and platelet-leukocyte conjugates (arrowhead in panel G) adhering to the wall of the coronary vessels in the graft of WT mice, whereas this was not seen in hearts transplanted in Gas6−/− mice (I). VCAM-1 (J,K) and ICAM-1 (L,M) up-regulated expression is reduced in Gas6−/− hearts transplanted into Gas6−/− mice (K,M) compared with WT cardiac grafts, dissected 3 days after transplantation into WT mice (J,L). Bar represents 100 μm in panels A and B, 50 μm in panels C-F and J-M, and 10 μm in panels G-I.
By contrast, when Gas6−/− hearts were grafted in Gas6−/− recipients, all 9 grafts survived for > 60 days, and appeared normal without signs of myocardial cell death, inflammation, or platelet/leukocyte sequestration (P < .05 vs WT in WT; Figure 4B,D,F,I). Consistent with our in vitro results, immunostaining confirmed that the expression of VCAM-1 and ICAM-1 by ECs was less prominent in Gas6−/− grafts compared with WT grafts (Figure 4J-M). Thus, loss of Gas6 delays graft destruction by attenuating tissue inflammation.
To study the relative importance of Gas6 production by the graft or recipient, we performed additional genetic crossover experiments. When Gas6−/− hearts were grafted into WT recipients, 70% of the grafts stopped beating within 7 to 12 days (N = 6; P < .05 vs Gas6−/− in Gas6−/−), with histologic signs of inflammation and myocardial cell death. Similar results were obtained when WT hearts were grafted in Gas6−/− recipients (N = 6; P < .05 vs Gas6−/− in Gas6−/−). These data suggest that Gas6 produced by both the donor heart (presumably produced by ECs) and host tissue (released by platelets or circulating in the plasma) determines graft destruction.
Discussion
This study provides novel evidence that Gas6 promotes EC activation and participates in the interactions between ECs, platelets, and leukocytes during inflammation. The principal findings of this study are: (1) Gas6 amplifies EC activation in response to inflammatory stimuli; (2) Gas6 promotes and accelerates the sequestration of circulating platelets and leukocytes in a P-selectin-dependent fashion; (3) Gas6 also promotes the formation and endothelial sequestration of circulating platelet-leukocyte conjugates; and (4) Gas6 promotes leukocyte extravasation, inflammation, and thrombosis in mouse models of endotoxinemia, vasculitis, and heart transplantation. Thus, these findings highlight a pivotal role for Gas6 in enhancing interactions between ECs and circulating cells during inflammation.
Our findings indicate that Gas6 promotes EC activation in response to the inflammatory stimuli TNF-α or LPS. Indeed, in response to TNF-α, loss or knockdown of Gas6 attenuates endothelial expression of adhesion receptors and the release of pro-inflammatory cytokines by both mouse and human ECs in vitro. Moreover, the impaired recruitment of circulating cells onto Gas6−/− ECs in vivo was likely attributable to absent expression of Gas6 by ECs because leukocyte recruitment was impaired when ECs in Gas6−/− mice were selectively activated.
The reduced activation of cultured Gas6−/− ECs translated in an impaired and delayed sequestration of circulating cells onto activated endothelium in the absence of Gas6 in vivo. Because loss of Gas6 reduced the recruitment of platelets, leukocytes, and platelet-leukocyte conjugates, we reasoned that the culprit might be reduced levels of P-selectin, an endothelial receptor, involved in the sequestration of these cells.38,42,43 Indeed, by intravital videomicroscopy and using a P-selectin inhibitor, we found that, on selective activation of the endothelium, Gas6 promotes and catalyzes the sequestration of circulating leukocytes and platelets in a P-selectin-dependent manner. Interestingly, GA failed to suppress the sequestration of platelets and leukocytes on Gas6−/− endothelium. Because ECs up-regulate their cell surface levels of P-selectin through rapid translocation of Weibel-Palade bodies, in which P-selectin and other molecules are stored,47 these findings suggest that release of P-selectin from these intracellular stores was impaired. Further support that loss of Gas6 impaired the exocytosis process of Weibel-Palade bodies was provided by our findings that the functional release of VWF (another molecule stored in Weibel-Palade bodies) was also defective in Gas6−/− mice, whereas the number of endothelial Weibel-Palade bodies was normal.
Apart from regulating the levels of endothelial P-selectin, Gas6 also regulated the expression of various other endothelial molecules. Indeed, in the absence of Gas6, activated ECs failed to up-regulate the expression of adhesion molecules (VCAM-1, ICAM-1) and the release of cytokines involved in the recruitment of leukocytes (IL-1β, IL-6). This in vitro finding translated in a reduction of leukocyte extravasation in Gas6−/− mice in vivo, in models of heart transplantation, endotoxinemia, and vasculitis. Indeed, histologic analysis of the transplanted hearts revealed that loss of Gas6 prevented the development of myocarditis and leukocyte infiltration in the grafts, consistent with previous reports on the role of Gas6 in graft rejection.52,53 In graft myocarditis and destruction, endothelial Gas6 plays a dominant role, as 70% of the cardiac grafts were rejected when Gas6−/− recipient mice received WT donor hearts, and careful high magnification microscopic analysis of the WT grafts revealed that platelets, leukocytes, and platelet-leukocyte conjugates often adhered to the wall of the coronary vessels. In addition, in the absence of Gas6, leukocyte extravasation was reduced after local and systemic administration of LPS. During endotoxinemia, loss of Gas6 also impaired the formation of platelet-leukocyte conjugates, which might have contributed to the reduced leukocyte extravasation and activation.11,34,49 Obviously, our findings do not exclude the possibility that Gas6 also affects leukocytes directly. Previous reports indeed showed that Gas6 and its receptors exert both positive and negative effects on leukocyte activation.14,27,28,31
Platelet Gas6 probably also plays a role in orchestrating inflammation because of its role in regulating the expression of platelet P-selectin. Indeed, previous reports showed that, in atherosclerosis and other inflammatory conditions, platelet P-selectin accelerates the extravasation of leukocytes into the inflamed tissue via the formation of platelet-leukocyte conjugates, thereby enabling binding of leukocytes to endothelial PSGL-1 or other sialoglycoproteins.34,41,42,44 P-selectin, expressed by activated and sequestered platelets, not only activates circulating leukocytes but can also increase EC activation and the levels of endothelial P-selectin.45 We found that, after systemic injection of LPS, the formation of platelet-leukocyte conjugates was impaired in Gas6−/− mice, which extends our previous report on reduced P-selectin levels in Gas6−/− platelets after activation.7 In addition, loss of Gas6 impaired the endothelial sequestration of platelets, leukocytes, and their conjugates, which likely contributed to the reduction of graft myocarditis and destruction in the heart transplantation model (in case Gas6−/− grafts were used). Thus, Gas6 regulates platelet activation, the levels of platelet P-selectin, and subsequent interactions with leukocytes and ECs during inflammation.
An outstanding question is how Gas6 acts at the molecular level. ECs release Gas6 constitutively, but binding of Gas6 to Axl is redundant for regulating ICAM-1 expression in baseline conditions. On activation by TNF-α, ECs do not produce increased amounts of Gas6 but respond to Gas6 (a process requiring the presence of Axl), possibly suggesting that Gas6 is only able to bind to Axl on activated ECs. An intriguing possibility is that the altered lipid composition of the plasma membrane in such conditions would favor an interaction of Gas6, via its gamma-carboxylated Gla-domain, to negatively charged phospholipids on activated EC surfaces, thereby increasing the affinity of Gas6 for its receptor.20 We also cannot exclude the (hypothetical) possibility that Gas6 may, in addition to its extracellular activity, act via “intracrine” mechanisms, as recently documented for VEGF.54 Obviously, future studies will be required to elucidate the precise molecular mechanism of Gas6.
Our findings might also have some medical implications. Expression analyses in the mouse indicate that ECs, platelets, and leukocytes produce and release Gas6, but Gas6 levels in human platelets are variable, from negligible37 to substantial.7 We show here that Gas6 also determines the response of human ECs to TNFα in vitro. Interestingly, Gas6 plasma levels are increased in endotoxinemic mice and septic patients (P.C., unpublished data, 2008).29,30 Besides being a biomarker of disease severity,29,30 it remains to be investigated whether the elevated plasma levels of Gas6 in response to sepsis are functionally relevant. Because treatment with activated protein C, the only available drug for sepsis, primarily protects the endothelium,55,56 our findings on the role of Gas6 in augmenting platelet-EC-leukocyte interactions and their activation profiles, suggest that inhibition of Gas6 might warrant further consideration as a novel strategy for the treatment of sepsis or transplantation-induced organ rejection.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Bouché, K. Brepoels, P. Chevron, N. Dai, M. De Mol, M. Deprez, B. Hermans, A. Janssen, S. Jansen, A. Manderveld, L. Notebaert, J. Souffreau, S. Terclavers, A. Vandenhoeck, P. Vandervoort, B. Vanwetswinkel, E. Weltens, S. Wyns (Leuven), and I. Nilsson (Malmo) for technical assistance.
This work was supported in part by the Swedish Research Council (07 143), by the Spanish Ministry of Science and Technology (grants SAF2004-07 539-C02 and BES2003-0230), by a grant from the Swiss National Foundation for Scientific research (PPOOB-106690/1), by a grant from the Belgian National Fund for Scientific Research in Flanders (FWO; G0265.01), by grant GOA2004/09 from the Concerted Research Activities, Belgium, and by an unrestricted Bristol-Myers-Squibb grant (P.C.). E.L. is a postdoctoral fellow of the Dr E. Dekker Program of the Dutch Heart Foundation (2000T041), M.T. is a postdoctoral research fellow of the Belgian Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT) and FWO.
Authorship
Contribution: M.T. designed and performed experiments, analyzed data, and participated in discussion and manuscript writing; L.B.-M., Y.L., and E.L. performed experiments and analyzed data; S.P. generated research tools, performed and designed experiments, and analyzed data; F.B., N.D.-T., and C.H. performed experiments and analyzed data; R.M., A.D.B., C.A., and M.L. performed experiments; M. Daemen participated in discussion; M. Dewerchin, F.L., and J.A. performed experiments and analyzed data; J.-M.H. performed experiments, analyzed data, and participated in discussion; M.W. designed experiments and participated in discussion; P.F.d.G. performed experiments, analyzed data, and participated in discussion; B.D. generated research tools and participated in discussion; P.C. conceived and initiated the study, designed experiments, analyzed data, participated in discussion and manuscript writing, and provided scientific direction; and M.F.H. and L.M. designed experiments, analyzed data, and participated in discussion and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Carmeliet, Center for Transgene Technology & Gene Therapy, Campus Gasthuisberg, Herestraat 49, Box 912, B-3000, Leuven, Belgium; e-mail: peter.carmeliet@med.kuleuven.be.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal