Abstract
Heparin cofactor II (HCII)–deficient mice form occlusive thrombi more rapidly than do wild-type mice following injury to the carotid arterial endothelium. Dermatan sulfate (DS) and heparan sulfate (HS) increase the rate of inhibition of thrombin by HCII in vitro, but it is unknown whether vascular glycosaminoglycans play a role in the antithrombotic effect of HCII in vivo. In this study, we found that intravenous injection of either wild-type recombinant HCII or a variant with low affinity for HS (K173H) corrected the abnormally short thrombosis time of HCII-deficient mice, while a variant with low affinity for DS (R189H) had no effect. When HCII was incubated with frozen sections of the mouse carotid artery, it bound specifically to DS in the adventitia. HCII was undetectable in the wall of the uninjured carotid artery, but it became concentrated in the adventitia following endothelial injury. These results support the hypothesis that HCII interacts with DS in the vessel wall after disruption of the endothelium and that this interaction regulates thrombus formation in vivo.
Introduction
Vascular injury allows circulating coagulation factor VIIa to interact with tissue factor.1 This interaction triggers a series of zymogen activation reactions, which lead to generation of the proteolytic enzyme thrombin. Thrombin promotes clot formation by converting fibrinogen to fibrin, by stimulating platelet aggregation and secretion, and by activating certain proteins (factors V, VIII, and XI) upstream in the coagulation cascade. Thrombin also has multiple effects on vascular endothelial cells, smooth muscle cells, and monocytes/macrophages that may be important in tissue repair or in the development of atherosclerotic or neointimal lesions.2
Thrombin can be inhibited by 2 serpins, antithrombin (AT) and heparin cofactor II (HCII), which are produced by the liver and circulate at micromolar concentrations in plasma.3 Both of these serpins inhibit thrombin at least 1000 times faster upon interaction with certain glycosaminoglycans. AT is activated by heparin or by heparan sulfate (HS) proteoglycans synthesized by vascular endothelial cells and is thought to produce an antithrombotic effect at the blood-endothelial interface.4 Patients with partial AT deficiency have an increased incidence of venous thromboembolism,5 and homozygous AT-deficient mice die in late gestation with evidence of fibrin deposition and a consumptive coagulopathy.6 Heparin and HS also bind and activate HCII, although with much lower affinity in comparison with AT.7 By contrast, purified dermatan sulfate (DS),7 as well as DS proteoglycans synthesized by fibroblasts,8,9 stimulate thrombin inhibition by HCII but not by AT; these findings support the hypothesis that HCII inhibits thrombin in the vessel wall after disruption of the endothelium. Patients with low plasma concentrations of HCII do not appear to be at increased risk for venous thrombosis,10 but they may be predisposed to development of atherosclerosis11 or neointima formation following angioplasty and stent placement.12,13
Homozygous HCII-deficient mice generated in our laboratory are born at the expected Mendelian frequency and do not show signs of spontaneous thrombosis despite the complete absence of HCII antigen and activity in plasma.14 When challenged with photochemical injury to the carotid arterial endothelium, however, they form occlusive thrombi faster than do wild-type mice. Furthermore, intravenous infusion of purified DS prior to injury prolongs the occlusion time of wild-type mice, but not of HCII−/− mice.15 These observations indicate that HCII inhibits thrombosis after arterial injury and that exogenous DS can augment the antithrombotic activity of HCII. Since HCII can inhibit thrombin in the absence of HS or DS, albeit very slowly, it remains uncertain whether the antithrombotic effect of HCII depends upon its interaction with endogenous glycosaminoglycans present in the vessel wall. To answer this question, we determined the ability of HCII variants with different affinities for HS and DS to correct the abnormally short thrombosis time of HCII−/− mice.
Methods
Reagents
Murine α-thrombin was purchased from Haematologic Technologies (Essex Junction, VT). DS from porcine intestinal mucosa was obtained from Sigma-Aldrich (St Louis, MO) and was treated with nitrous acid to remove contaminating heparin prior to use.16 Heparin was obtained from Baxter Healthcare (Deerfield, IL). Tosyl-Gly-Pro-Arg-p-nitroanilide (Chromozym TH) was purchased from Roche Applied Science (Indianapolis, IN). Chondroitin B-lyase, chondroitin ABC-lyase, Flavobacterium heparitinase I, and mouse monoclonal ΔDi-4S and ΔHS antibodies were purchased from Seikagaku (Tokyo, Japan). Affinity-purified goat anti-HCII IgG was obtained from Affinity Biologicals (Hamilton, ON). Biotinylated goat anti–mouse IgG, biotinylated rabbit anti–goat IgG, and avidin:biotinylated peroxidase complex were obtained from Vector Laboratories (Burlingame, CA). 3,3′-Diaminobenzidine (DAB500Pack) was purchased from Biocare Medical (Walnut Creek, CA).
Mice
All experimental protocols were approved by the Animal Studies Committee of Washington University School of Medicine. HCII-deficient mice were generated by homologous recombination in 129/SvJ-derived embryonic stem cells14 and were backcrossed 15 or more times with inbred C57BL/6 mice purchased from Taconic (Germantown, NY). HCII+/+ mice from double heterozygous matings or wild-type C57BL/6 mice from Taconic served as controls.
Recombinant HCII
Recombinant human HCII variants (rHCIIWT, rHCIIR189H, and rHCIIK173Q) were expressed in Escherichia coli as previously described.17 Recombinant proteins were isolated from the cell lysates by immunoaffinity chromatography using sheep anti-HCII IgG (Affinity Biologicals) according to the manufacturer's protocol and further purified on a Mono Q (Pharmacia Biotech, Piscataway, NJ) ion exchange column. Purity was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% polyacrylamide gels stained with Coomassie blue. The protein concentration was determined from the absorbance at 280 nm using the extinction coefficient 77 000 M−1cm−1.18
Inhibition of murine thrombin by rHCII
Recombinant HCII (185 nM), murine α-thrombin (14 nM), and DS or heparin at various concentrations were incubated together at room temperature in 0.1 mL of 50 mM Tris-HCl, 150 mM NaCl, 1 mg/mL poly(ethylene glycol) 8000, pH 7.4 (TSP buffer) in a polystyrene cuvette. Thrombin was added last to initiate the reaction. After exactly 60 seconds, 0.5 mL tosyl-Gly-Pro-Arg-p-nitroanilide (100 μM in TSP buffer) was added and the absorbance at 405 nm was determined continuously for 100 seconds. The rate of change of absorbance was proportional to thrombin activity.
Arterial thrombosis model
Carotid artery thrombosis was induced as described previously.14 Adult male and female mice (10-19 weeks of age; 20-28 g body weight) were anesthetized with intraperitoneal sodium pentobarbital, secured in the supine position, and placed under a dissecting microscope. The right common carotid artery was isolated through a midline cervical incision, and an ultrasonic flow probe (Model 0.5 VB; Transonic Systems, Ithaca, NY) was applied. A 1.5-mW, 540-nm laser beam (Melles Griot, Carlsbad, CA) was applied to the artery from a distance of 6 cm. Rose bengal dye (3′,4′,5′,6′-tetrachloro-2,4,5,7-tetraiodofluorescein; Fisher Scientific, Fair Lawn, NJ) at a dose of 50 mg/kg body weight was then injected into the lateral tail vein, and blood flow was monitored continuously. The occlusion time was taken as the interval between injection of rose bengal dye and complete and stable (> 5 minutes) cessation of flow.
Recombinant HCII was dialyzed into 0.15 M NaCl, sterilized by filtration through a 0.2-μm filter, and injected into the lateral tail vein 15 minutes before injection of rose bengal dye. The total volume of material injected intravenously did not exceed 10% of the estimated blood volume of the mouse.
Although males and females were not evenly distributed among the experimental groups in this study, a pooled analysis of C57BL/6 (N ≥ 10) mice from several of our previous studies revealed no sex differences in thrombosis times for either HCII genotype: HCII+/+ (57.2 ± 6.7 minutes for males [n = 11] vs 62.2 ± 7.6 minutes for females [n = 10]; P = .125) or HCII−/− (35.8 ± 4.1 minute for males [n = 15] vs 37.9 ± 4.6 minutes for females [n = 8]; P = .215).
Clearance of rHCII
Recombinant HCII was prepared and injected into anesthetized HCII−/− mice as described under “Arterial thrombosis model.” At various times after injection, blood from a tail tip incision was collected into one-tenth volume of 0.105 M sodium citrate, and plasma was prepared by centrifugation. Plasma HCII antigen was determined by enzyme-linked immunosorbent assay (ELISA) as previously described.19
Immunohistochemical localization of DS and HS
Frozen sections of the carotid artery were fixed with absolute ethanol for 20 minutes at − 20°C and then treated with 0.3% (vol/vol) horse serum and 0.3% (vol/vol) H2O2 in phosphate-buffered saline, pH 7.4 (PBS), for 5 minutes to eliminate endogenous peroxidase activity. The sections were then incubated overnight at 37°C with chondroitin B-lyase or Flavobacterium heparitinase under conditions previously described.20 Incubations with buffer alone served as controls. Antibody staining was performed using the Mouse on Mouse Immunodetection Kit (Vector Laboratories) according to the manufacturer's protocol. Mouse monoclonal antibodies ΔDi-4S and ΔHS were each used at a concentration of 2 μg/mL; these antibodies recognize the Δ4,5uronic acid→GalNAc4SO3 disaccharide generated from DS and the Δ4,5uronic acid generated from HS, respectively (Table 1). The bound antibody was detected by incubation with biotinylated goat anti–mouse IgG, avidin:biotinylated peroxidase complex, and 3,3′-diaminobenzidine, which generates a brown color. The sections were counterstained with Mayer hematoxylin and examined with a Leica DMLS microscope (Leica Microsystems, Bannockburn, IL) equipped with 10× ocular and 40× objective lenses. Images were acquired with a QImaging MicroPublisher digital camera and QCapture version 1.1.4 software (QImaging, Burnaby, BC).
Specificities of lyases
| Lyase (reference) . | Substrate(s) . | Glycosidic linkage(s) cleaved . |
|---|---|---|
| Chondroitin B-lyase (Michelacci et al21 ) | DS | GalNAc4SO3→IdoA |
| Chondroitin ABC-lyase (Oike et al22 ) | DS, C4S, C6S | GalNAc4SO3 and/or 6SO3→UA;GalNAc4SO3→UA2SO3 |
| Flavobacterium heparitinase (Linker and Hovingh23 ) | HS | GlcNAc6SO3→UA;GlcNSO3→UA;GlcNSO36SO3→UA |
| Lyase (reference) . | Substrate(s) . | Glycosidic linkage(s) cleaved . |
|---|---|---|
| Chondroitin B-lyase (Michelacci et al21 ) | DS | GalNAc4SO3→IdoA |
| Chondroitin ABC-lyase (Oike et al22 ) | DS, C4S, C6S | GalNAc4SO3 and/or 6SO3→UA;GalNAc4SO3→UA2SO3 |
| Flavobacterium heparitinase (Linker and Hovingh23 ) | HS | GlcNAc6SO3→UA;GlcNSO3→UA;GlcNSO36SO3→UA |
C4S indicates chondroitin 4-sulfate; C6S, chondroitin 6-sulfate; GalNAc4SO3, N-acetylgalactosamine 4-O-sulfate; GlcNAc6SO3, N-acetylglucosamine 6-O-sulfate; GlcNSO3, N-sulfoglucosamine; GlcNSO36SO3, N-sulfoglucosamine 6-O-sulfate; IdoA, iduronic acid; and UA, hexuronic acid (glucuronic or iduronic acid).
Immunohistochemical localization of HCII
Frozen sections of the carotid artery were fixed with absolute ethanol, treated with 0.5% (vol/vol) H2O2, and incubated for 30 minutes with blocking buffer containing 3% (vol/vol) bovine serum albumin and 1 drop/mL rabbit serum in phosphate-buffered saline (PBS), pH 7.4. The sections were then incubated for 1 hour at room temperature with goat anti–HCII IgG diluted to 2 μg/mL in blocking buffer. The bound antibody was detected by incubation with biotinylated rabbit anti–goat IgG, avidin:biotinylated peroxidase complex, and 3,3′-diaminobenzidine. The sections were counterstained with Mayer hematoxylin and examined by microscopy as described under “Immunohistochemical localization of DS and HS.”
Binding of rHCII to arterial sections
Frozen arterial sections obtained from HCII−/− mice were processed as described under “Immunohistochemical localization of HCII” with the following modifications: Prior to the addition of goat anti–HCII IgG, the sections were incubated for 1 hour at room temperature with 20 μg/mL rHCII (wild-type, K173Q, or R189H) in PBS and then rinsed twice with PBS. For some experiments, the sections were preincubated for 2 hours at 37°C with chondroitin ABC-lyase as previously described20 before the addition of rHCII.
Statistical analysis
Data are expressed as the mean values plus or minus 1 standard deviation (SD). Statistical significance was determined with the Student 2-tailed t test for independent samples. P values less than .05 are considered significant.
Results
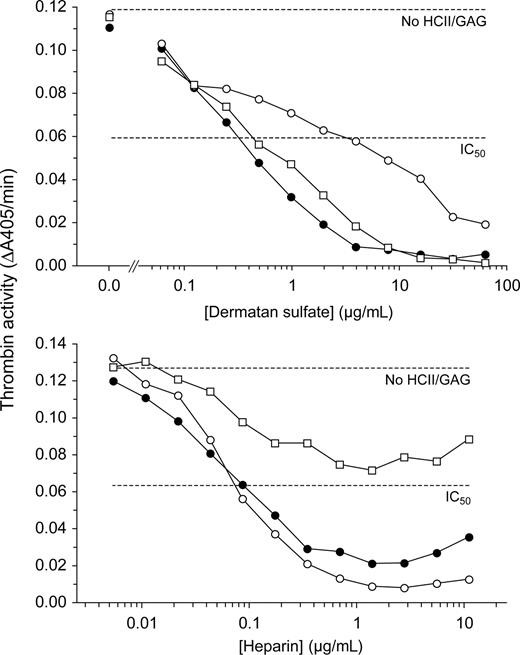
HCII−/− mice were reconstituted with wild-type recombinant human HCII (rHCIIWT) or with variants containing point mutations previously shown to reduce the affinity of binding to either DS (rHCIIR189H) or heparin (rHCIIK173Q).24,25 Neither mutation alters the kinetics of inhibition of thrombin by HCII in the absence of DS or heparin.17 Each variant was expressed in E coli and purified to more than 95% homogeneity as assessed by SDS-PAGE. Although these variants were previously characterized with respect to inhibition of human thrombin,17 their ability to inhibit murine thrombin has not been established. Figure 1 shows dose-response curves for inhibition of murine thrombin by each HCII variant in the presence of DS or heparin. Comparison of the IC50 values indicates that approximately 10-fold higher concentrations of DS are necessary to promote thrombin inhibition by rHCIIR189H in comparison with rHCIIWT, whereas rHCIIWT and rHCIIK173Q are activated by similar concentrations of DS. Conversely, heparin activates rHCIIWT and rHCIIR189H at similar concentrations but is much less effective in activation of rHCIIK173Q. These results are similar to those obtained previously with human thrombin.17
Inhibition of murine thrombin by rHCII variants. Murine thrombin (14 nM) was incubated for 60 seconds with 185 nM human rHCII (WT, ●; R189H, ○; K173Q, □) and either DS or heparin at the final concentrations shown. Residual thrombin activity was then assayed with a chromogenic substrate (ΔA405/min). No HCII/GAG indicates thrombin activity in the absence of HCII and glycosaminoglycan; IC50, 50% of initial thrombin activity.
Inhibition of murine thrombin by rHCII variants. Murine thrombin (14 nM) was incubated for 60 seconds with 185 nM human rHCII (WT, ●; R189H, ○; K173Q, □) and either DS or heparin at the final concentrations shown. Residual thrombin activity was then assayed with a chromogenic substrate (ΔA405/min). No HCII/GAG indicates thrombin activity in the absence of HCII and glycosaminoglycan; IC50, 50% of initial thrombin activity.
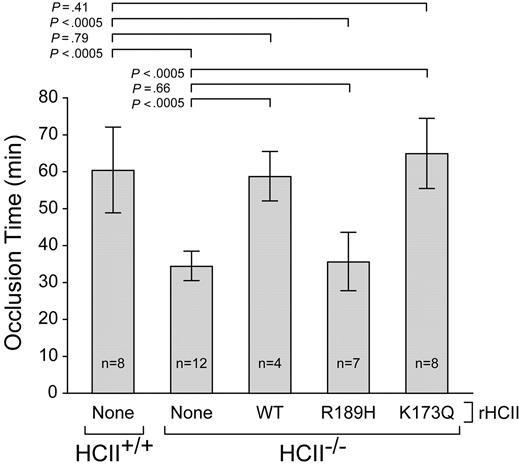
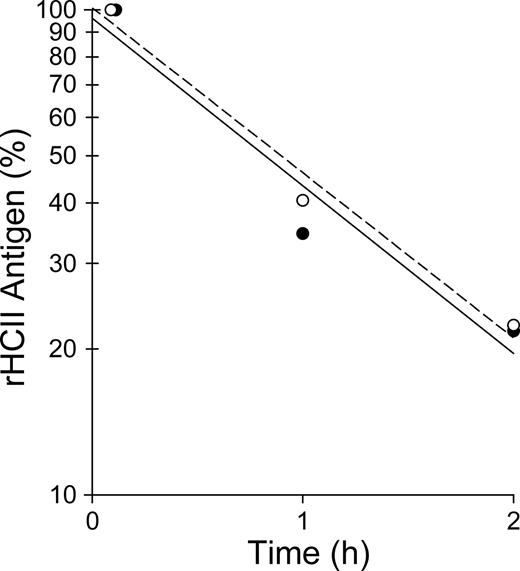
HCII−/− mice were injected intravenously with a single dose of rHCII calculated to achieve a plasma concentration of 0.125 μM, which is approximately half the concentration of HCII in wild-type mouse plasma.14,26 Control animals were given an equal volume of saline. After a 15-minute equilibration period, endothelial injury was triggered by photochemical activation of rose bengal dye in the common carotid artery. The interval between the onset of injury and complete thrombotic occlusion of the artery was then determined (Figure 2). In agreement with our previous results,14,15 the occlusion times of HCII−/− mice given saline were significantly shorter than those of HCII+/+ mice (34 ± 4 minutes vs 60 ± 12 minutes). HCII−/− mice reconstituted with either rHCIIWT or rHCIIK173Q had occlusion times (59 ± 7 minutes and 65 ± 9 minutes, respectively) that were similar to those of HCII+/+ mice. By contrast, HCII−/− mice reconstituted with rHCIIR189H had occlusion times (36 ± 8 minutes) that were indistinguishable from those of HCII−/− mice given saline. We considered the possibility that rHCIIR189H failed to normalize the occlusion time because it was cleared faster than rHCIIWT from the mouse circulation. Therefore, we injected each protein into HCII−/− mice, obtained blood samples 5 minutes, 1 hour, and 2 hours after injection, and determined the plasma HCII antigen by ELISA. Both rHCIIR189H and rHCIIWT were cleared with half-lives of approximately 0.9 hour (Figure 3). These results indicate that the DS-binding site in HCII is required for antithrombotic activity in vivo and raise the possibility that HCII interacts with DS present in the arterial wall. The results of this experiment also suggest that interaction of HCII with heparin-like molecules (eg, HS) may not be required for antithrombotic activity.
Thrombotic occlusion times after photochemical injury of the carotid artery. HCII+/+ or HCII−/− mice were injected intravenously with saline (none) or purified rHCII (WT, R189H, or K173Q) 15 minutes before injection of rose bengal dye. The dose of rHCII was calculated to achieve a plasma concentration of 0.125 μM. The bars indicate the means plus or minus SD.
Thrombotic occlusion times after photochemical injury of the carotid artery. HCII+/+ or HCII−/− mice were injected intravenously with saline (none) or purified rHCII (WT, R189H, or K173Q) 15 minutes before injection of rose bengal dye. The dose of rHCII was calculated to achieve a plasma concentration of 0.125 μM. The bars indicate the means plus or minus SD.
Clearance of rHCII from the mouse circulation. HCII−/− mice were injected intravenously with rHCIIWT (●, —) or rHCIIR189H (○, ----) as in Figure 2. Blood samples were collected 5 minutes, 1 hour, and 2 hours after injection, and the plasma HCII antigen was determined in duplicate by ELISA. HCII antigen values were normalized to the 5-minute point (100%). The half-life was calculated from the slope of the line fit to log(HCII antigen) versus time and was approximately 0.9 hour for each protein.
Clearance of rHCII from the mouse circulation. HCII−/− mice were injected intravenously with rHCIIWT (●, —) or rHCIIR189H (○, ----) as in Figure 2. Blood samples were collected 5 minutes, 1 hour, and 2 hours after injection, and the plasma HCII antigen was determined in duplicate by ELISA. HCII antigen values were normalized to the 5-minute point (100%). The half-life was calculated from the slope of the line fit to log(HCII antigen) versus time and was approximately 0.9 hour for each protein.
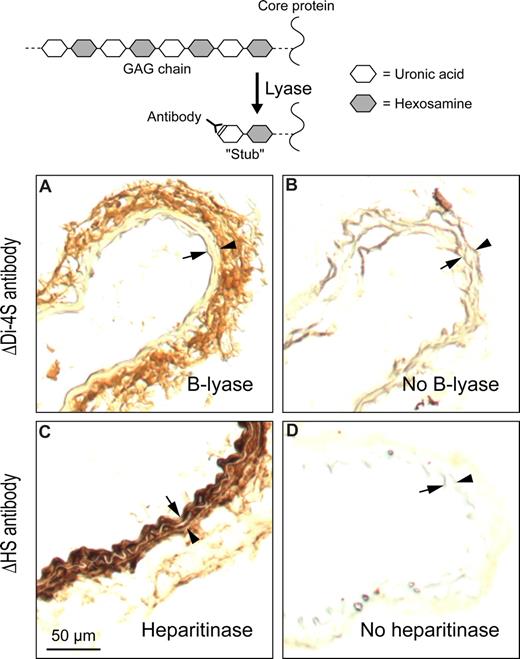
Immunohistochemical methods were used to determine the location of DS in the mouse carotid artery. Frozen sections were treated with chondroitin B-lyase, which cleaves specific glycosidic linkages in DS (Table 1), leaving behind oligosaccharide “stubs” with unsaturated Δ4,5-hexuronic acid residues at their nonreducing termini. The sections were then stained with an antibody (ΔDi-4S) that recognizes the new epitope.27 DS was readily detected in the adventitia of the carotid artery but not in the intima or media (Figure 4A). Additional sections were treated with Flavobacterium heparitinase, which cleaves HS, and stained with an appropriate antibody (ΔHS).28 In contrast to DS, HS was detected mainly in the intima and media of the carotid artery (Figure 4C). Frozen sections that were not treated with enzyme showed minimal background staining with both antibodies (Figure 4B,D).
Localization of DS and HS in uninjured carotid arteries of wild-type mice. Frozen sections were treated with chondroitin B-lyase (A), Flavobacterium heparitinase (C), or buffer alone (B,D) and then incubated with monoclonal antibodies ΔDi-4S (A,B) or ΔHS (C,D). Bound monoclonal antibodies were detected with a peroxidase-conjugated secondary antibody. Arrow indicates internal elastic lamina; arrowhead, external elastic lamina. DS was present primarily in the adventitia (A) and HS in the intima/media (C).
Localization of DS and HS in uninjured carotid arteries of wild-type mice. Frozen sections were treated with chondroitin B-lyase (A), Flavobacterium heparitinase (C), or buffer alone (B,D) and then incubated with monoclonal antibodies ΔDi-4S (A,B) or ΔHS (C,D). Bound monoclonal antibodies were detected with a peroxidase-conjugated secondary antibody. Arrow indicates internal elastic lamina; arrowhead, external elastic lamina. DS was present primarily in the adventitia (A) and HS in the intima/media (C).
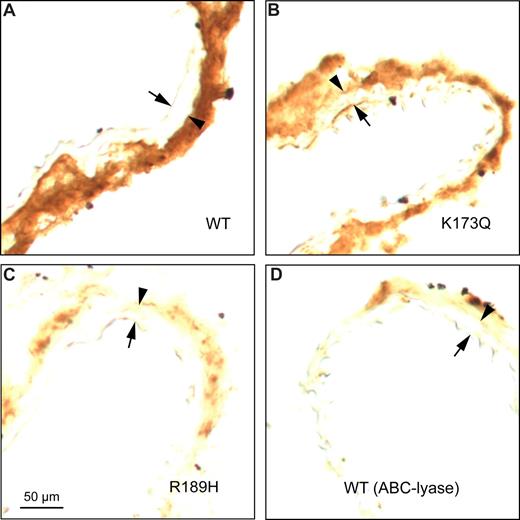
To identify HCII-binding sites in the arterial wall, frozen sections of carotid arteries from HCII−/− mice were incubated with purified rHCII, rinsed to remove excess unbound protein, and stained with an anti-HCII antibody. Figure 5A shows that binding of rHCIIWT was restricted to the adventitia. The binding sites appear to be composed predominantly of DS molecules, since binding of rHCIIR189H was diminished in comparison with rHCIIWT or rHCIIK173Q (Figure 5A-C). Furthermore, pretreatment of frozen sections with chondroitin ABC-lyase, which degrades DS but not HS (Table 1), greatly reduced the amount of rHCIIWT bound (Figure 5D).
Binding of HCII to carotid arterial sections in vitro. Frozen sections were incubated for 1 hour at room temperature with 20 μg/mL rHCIIWT (A), rHCIIK173Q (B), or rHCIIR189H (C) in PBS. After 2 rinses with buffer, the sections were stained with a polyclonal goat anti-HCII IgG. The section in panel D was treated with chondroitin ABC-lyase to degrade DS prior to incubation with rHCIIWT. Arrow indicates internal elastic lamina; arrowhead, external elastic lamina. rHCIIWT and rHCIIK173Q bound strongly to sites in the adventitia.
Binding of HCII to carotid arterial sections in vitro. Frozen sections were incubated for 1 hour at room temperature with 20 μg/mL rHCIIWT (A), rHCIIK173Q (B), or rHCIIR189H (C) in PBS. After 2 rinses with buffer, the sections were stained with a polyclonal goat anti-HCII IgG. The section in panel D was treated with chondroitin ABC-lyase to degrade DS prior to incubation with rHCIIWT. Arrow indicates internal elastic lamina; arrowhead, external elastic lamina. rHCIIWT and rHCIIK173Q bound strongly to sites in the adventitia.
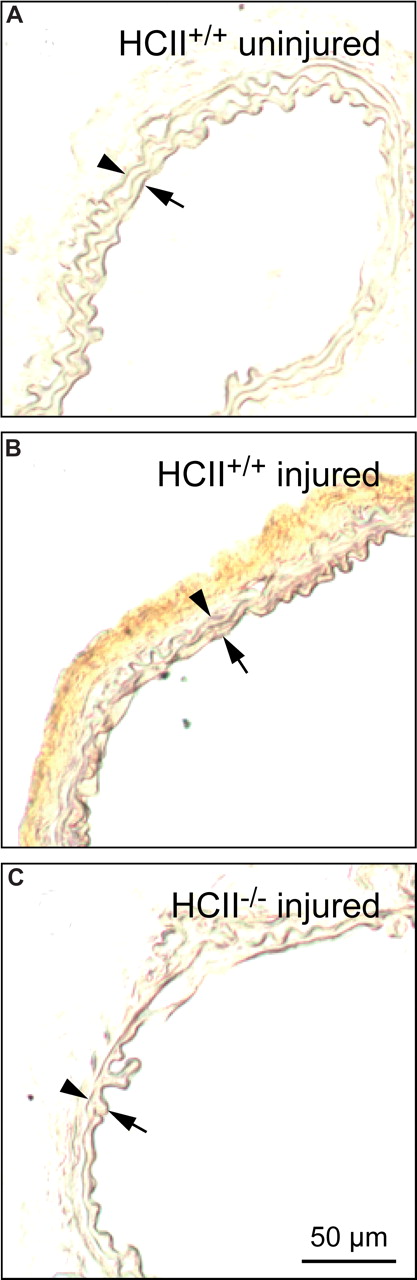
Little or no endogenous HCII antigen was detectable in carotid arteries harvested from HCII+/+ mice prior to photochemical injury (Figure 6A). By contrast, HCII antigen was clearly present in the adventitia 30 minutes after the onset of photochemical injury (Figure 6B). In control experiments, staining for HCII antigen was negative in injured carotid arteries from HCII−/− mice (Figure 6C). Figure 7 shows the distribution of HCII antigen in photochemically injured carotid arteries of HCII−/− mice that had been reconstituted by intravenous injection of rHCII. Thirty minutes after injury, both rHCIIWT and rHCIIK173Q (Figure 7A,B) were present in the adventitia at much higher levels in comparison with rHCIIR189H (Figure 7C). Staining for HCII antigen was negative in HCII−/− mice preinjected with saline (Figure 7D).
Distribution of endogenous HCII before and after injury. Frozen sections of carotid arteries harvested from HCII+/+ mice before (A) or 30 minutes after (B) the onset of injury were stained with a polyclonal goat anti–HCII IgG. No HCII antigen was detected in the injured carotid artery of a control (HCII−/−) mouse (C). Arrow indicates internal elastic lamina; arrowhead, external elastic lamina.
Distribution of endogenous HCII before and after injury. Frozen sections of carotid arteries harvested from HCII+/+ mice before (A) or 30 minutes after (B) the onset of injury were stained with a polyclonal goat anti–HCII IgG. No HCII antigen was detected in the injured carotid artery of a control (HCII−/−) mouse (C). Arrow indicates internal elastic lamina; arrowhead, external elastic lamina.
Distribution of rHCII variants after arterial injury. HCII−/− mice were injected with rHCIIWT (A), rHCIIK173Q (B), rHCIIR189H (C), or saline (D) and then subjected to photochemical injury as described in the legend to Figure 2. Frozen sections of arteries harvested 30 minutes after the onset of injury were stained with a polyclonal goat anti–HCII IgG. Arrow indicates internal elastic lamina; arrowhead, external elastic lamina.
Distribution of rHCII variants after arterial injury. HCII−/− mice were injected with rHCIIWT (A), rHCIIK173Q (B), rHCIIR189H (C), or saline (D) and then subjected to photochemical injury as described in the legend to Figure 2. Frozen sections of arteries harvested 30 minutes after the onset of injury were stained with a polyclonal goat anti–HCII IgG. Arrow indicates internal elastic lamina; arrowhead, external elastic lamina.
Discussion
In this study, we have shown that HCII binds to DS in the adventitia of the mouse carotid artery and that the antithrombotic effect of HCII depends upon this interaction. These conclusions are supported by the following observations: (1) DS is localized predominantly in the arterial adventitia; by contrast, HS is concentrated in the intima and media. (2) Binding of purified HCII to frozen sections of the carotid artery is limited to the adventitia. (3) The HCII-binding sites are composed of DS, since binding is abolished either by treatment of the arterial section with chondroitin ABC-lyase or by a mutation in HCII (R189H) that decreases its affinity for DS; binding is unaffected by a mutation (K173Q) that decreases the affinity of HCII for HS. (4) Little or no endogenous HCII can be detected in the walls of intact carotid arteries harvested from wild-type mice. (5) Following injury to the carotid arterial endothelium in vivo, but preceding the formation of an occlusive thrombus, HCIIWT or HCIIK173Q (but not HCIIR189H) become concentrated in the adventitia. (6) Finally, reconstitution of HCII−/− mice by intravenous injection of HCIIWT or HCIIK173Q (but not HCIIR189H) lengthens the time required for formation of an occlusive thrombus after carotid injury. Taken together, these results support the long-standing hypothesis that DS in the vessel wall binds HCII and thereby catalyzes the inhibition of thrombin in vivo.7 The details of this interaction remain speculative, however, because we do not yet have direct evidence that inhibition of thrombin by HCII bound to DS occurs in the adventitia.
Previous studies showed that vascular DS has anticoagulant activity in vitro. Tovar et al29 reported that similar amounts of DS and HS are present in the human aorta (approximately 1.0-1.3 μg hexuronic acid per milligram dry weight). They noted that DS isolated from the aorta is several times more potent than HS in its ability to prolong the activated partial thromboplastin time and, therefore, concluded that DS is the major anticoagulant glycosaminoglycan in the walls of large blood vessels. Since DS does not prolong the clotting time of HCII-depleted plasma,30 its anticoagulant effect depends upon the presence of HCII. Current evidence suggests that the anticoagulant activity of DS also depends upon biosynthetic reactions in which some of the iduronic acid→N-acetylgalactosamine 4-O-sulfate subunits are modified either by 2-O-sulfation of the iduronic acid or by 6-O-sulfation of the N-acetylgalactosamine to generate HCII-binding sites.31,32 The extent of these biosynthetic modifications varies in different tissues and may determine the sites at which HCII becomes activated.
The observation that DS is localized primarily in the adventitia of the mouse carotid artery suggests that fibroblasts are responsible for its biosynthesis. In a previous study, we found that fibroblast monolayers stimulate the thrombin-HCII reaction.8 Analysis of extracts from these cells indicated that a small DS-containing proteoglycan was primarily responsible for activation of HCII. Although the fibroblast proteoglycan was not identified, biglycan and decorin are possible candidates, since both of these proteoglycans isolated from other tissues have been shown to activate HCII.9 Vascular smooth muscle cells in culture also stimulate the thrombin-HCII reaction.8 However, the colocalization of HCII with DS in the adventitia of the carotid artery after photochemical injury suggests that smooth muscle cells, which reside in the media, may not participate in activation of HCII during experimental thrombosis.
In our experiments, thrombosis was induced by singlet oxygen generated from rose bengal dye in the bloodstream upon transillumination of the vessel with green light. This procedure causes rapid detachment of the arterial endothelium, leaving the elastic laminae and underlying smooth muscle cells intact.33 We did not detect HCII in the wall of the mouse carotid artery prior to injury, but after injury HCII was readily detectable in the adventitia. Thrombin generation may be initiated in the adventitia, which is the predominant site of expression of tissue factor in normal blood vessels.34-36 Thus, HCII bound to DS in the adventitia would be well positioned to regulate thrombin activity in the injured vessel.
Clearance studies of HCII in humans, baboons, and rabbits suggest that approximately 40% to 60% of the protein distributes into a noncirculating compartment within hours after injection and that the circulating pool is cleared with a half-life of 1.6 to 2.5 days.37-39 Although HCII levels have not been measured in interstitial fluid, lymph collected from rabbit hind limbs contains other hemostatic proteins at substantial concentrations, which are roughly in inverse proportion to the proteins' molecular weights.40 Since AT, which is similar to HCII in molecular weight, is present in hind limb lymph at approximately 40% of its plasma concentration, it is likely that HCII would also be found in lymph. These and other plasma proteins appear to cross the endothelium by transcytosis mainly at the level of the capillary or postcapillary venule.41 Relatively little transcytosis is thought to occur across the endothelia of larger vessels, which is consistent with our inability to detect HCII in the wall of the carotid artery before endothelial injury. Other investigators have reported, however, that HCII is present in the intima of normal human arteries42 and that radiolabeled HCII is taken up into the intima or media of the intact rabbit aorta.37 Nevertheless, it is clear from our immunohistochemical experiments that much larger amounts of HCII enter the arterial wall following endothelial injury.
The results of this study support a model in which HCII becomes activated by binding to DS in the arterial wall after disruption of the endothelium. This model is consistent with the observation that HCII−/− mice do not show signs of spontaneous thrombosis but develop thrombi more rapidly than wild-type mice after arterial injury14 ; furthermore, the ability of recombinant HCII to restore the thrombosis time of HCII−/− mice to normal correlates with its ability to bind to DS in the arterial adventitia. In comparison, AT is thought to be activated physiologically by HS proteoglycans associated with intact endothelial cells.4 Homozygous mutations in the HS-binding site of AT lead to spontaneous thrombosis in both humans and mice,43,44 providing support for this hypothesis. However, it has been difficult to show that binding of AT to endothelial HS mediates its antithrombotic effect. For example, binding of 125I-labeled AT to the mouse carotid arterial endothelium is absent in mice lacking the biosynthetic enzyme glucosaminyl 3-O-sulfotransferase-1, but these mice do not have evidence of spontaneous thrombosis nor do they have accelerated thrombosis in a carotid injury model.45 These results might be explained if low levels of 3-O-sulfation produced by another enzyme (eg, glucosaminyl 3-O-sulfotransferase-5) are sufficient to activate AT physiologically.46 Alternatively, AT may be activated by unmodified HS, which binds to AT with a much lower affinity but is present at much higher concentrations in blood vessels relative to 3-O-sulfated HS.47
In a previous study, we showed that intravenous administration of porcine skin dermatan sulfate prolongs the thrombosis time in mice and that this effect is HCII dependent.15 We noticed that the onset of antithrombotic activity was delayed when lower doses of DS were administered. Furthermore, in experiments in which we varied the interval between administration of DS and the onset of endothelial injury, we found that the antithrombotic effect persisted at times when most of the DS had disappeared from the bloodstream. These observations suggested that exogenous DS expresses antithrombotic activity after being transferred from the plasma to sites in the vessel wall. Although we have not yet identified these sites or quantified the uptake of DS, the results are consistent with our current finding that DS endogenously present in the vessel wall can bind and presumably activate HCII.
In conclusion, we have demonstrated that vascular DS mediates the antithrombotic effect of HCII in vivo. These results lead to the hypothesis that disorders of DS metabolism might contribute to vascular pathology by diminishing the activity of HCII. Recent clinical studies suggest that low levels of HCII promote in-stent restenosis12,13 and, in certain populations, atherogenesis.11,19 In mice, HCII deficiency causes accelerated atherosclerosis and vascular remodeling,48,49 and intravenous administration of DS reduces the degree of neointima formation.49 Further investigation will be required to determine whether interactions between HCII and endogenous DS play a role in these pathologic processes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Benjamin Tollefsen and Susan Beecher for their assistance in preparing the recombinant proteins and managing the mouse colony.
This work was supported by grants from the National Institutes of Health (HL55520) and the Edward Mallinckrodt Jr Foundation.
National Institutes of Health
Authorship
Contribution: L.H., T.K.G., C.P.V., and D.M.T. designed research and performed experiments; D.M.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Douglas M. Tollefsen, Hematology Division, Campus Box 8125, Washington University Medical School, 660 South Euclid Ave, St Louis, MO 63110; e-mail: tollefsen@im.wustl.edu.
References
Author notes
*L.H. and T.K.G. contributed equally to the paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal