Abstract
Angiopoietin-1 (Ang-1), ligand for the endothelial cell–specific Tie-2 receptors, promotes migration and proliferation of endothelial cells, however, whether these effects are promoted through the release of a secondary mediator remains unclear. In this study, we assessed whether Ang-1 promotes endothelial cell migration and proliferation through the release of interleukin-8 (IL-8). Ang-1 elicited in human umbilical vein endothelial cells (HUVECs) a dose- and time-dependent increase in IL-8 production as a result of induction of mRNA and enhanced mRNA stability of IL-8 transcripts. IL-8 production is also elevated in HUVECs transduced with retroviruses expressing Ang-1. Neutralization of IL-8 in these cells with a specific antibody significantly attenuated proliferation and migration and induced caspase-3 activation. Exposure to Ang-1 triggered a significant increase in DNA binding of activator protein-1 (AP-1) to a relatively short fragment of IL-8 promoter. Upstream from the AP-1 complex, up-regulation of IL-8 transcription by Ang-1 was mediated through the Erk1/2, SAPK/JNK, and PI-3 kinase pathways, which triggered c-Jun phosphorylation on Ser63 and Ser73. These results suggest that promotion of endothelial migration and proliferation by Ang-1 is mediated, in part, through the production of IL-8, which acts in an autocrine fashion to suppress apoptosis and facilitate cell proliferation and migration.
Introduction
Angiopoietin-1 (Ang-1) and its receptor, Tie-2, are rapidly emerging as important modulators of normal and pathological angiogenesis. In mice embryos, deletion of the Ang-1 gene produces lethality, with major defects in the vascular endothelium.1 In cultured endothelial cells (ECs), Ang-1 inhibits apoptosis and inflammatory responses and promotes differentiation, sprouting, and migration.2 In vivo, Ang-1 enhances collateral vessel formation in ischemia-induced angiogenesis.3 Exposure of ECs to Ang-1 triggers the autophosphorylation of Tie-2 receptors and the activation of downstream pathways, including the PI-3 kinase and 3 members of the mitogen activated protein kinases (MAPKs) (Erk1/2, p38, and SAPK/JNK).2,4 Whether these pathways mediate Tie-2 signaling through the release of soluble mediators remains unclear. Recently, 2 studies have reported that Ang-1 promotes smooth muscle recruitment through the release of heparin-binding epidermal growth factor (EGF)–like growth factor (HB-EGF), and hepatocyte growth factor (HGF) from ECs.5,6 It remains unclear whether or not these secreted mediators, or as yet unknown factors, modulate Ang-1 effects on EC proliferation, apoptosis, and migration
We recently evaluated the transcriptome of human umbilical vein endothelial cells (HUVECs) exposed to Ang-1 using microarrays.7 Exposure to Ang-1 resulted in induction of 86 genes that are involved in EC proliferation, differentiation, migration, and survival. In addition, Ang-1 elicited a significant decline in the expression of 49 genes, most of which are involved in cell-cycle arrest, apoptosis, and suppression of transcription. Our results also revealed that the only proangiogenesis chemokine that was significantly induced by Ang-1 exposure in HUVECs was interleukin-8 (IL-8). IL-8 is a CXC chemokine with high binding affinity for CXCR1 and CXCR2 receptors, both of which are abundantly expressed on ECs. These results were surprising, since Ang-1 exposure attenuates thrombin-induced IL-8 production in ECs.8 IL-8 is mainly expressed by leukocytes, fibroblasts, ECs, and tumor cells and plays important roles in chemoattraction, inflammation, and tumor angiogenesis.9 Others have reported that IL-8 directly induces EC migration, proliferation, and tube formation.10 These biological effects of IL-8, and our observation of an increase in IL-8 mRNA in HUVECs exposed to Ang-1, raise the possibility that some of the biological effects of Ang-1 on cultured ECs may be mediated indirectly through the release of IL-8, which might act in an autocrine fashion on CXCR1 and CXCR2 receptors expressed on the surface of ECs. Accordingly, the main focus of the current study is to test the hypothesis that Ang-1–induced EC survival, migration, and proliferation are mediated, in part, through increased IL-8 production. We also investigated in this study the signaling pathways and the transcription factors involved in the regulation of IL-8 production by the Ang-1/Tie 2 receptor pathway.
Methods
Detailed methods can be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell culture
HUVECs were maintained in culture, as described.11 Full-length murine Ang-1 cDNA was cloned into a retrovirus vector (MSCV-pac) and was transfected into Ampho Phoenix packaging cells. Viral supernatants from these cells were used to transduce HUVECs in multiple rounds of infection. Transduced cells were then selected in puromycin to produce MSCV-HUVECs and MSCV-Ang-1-HUVECs. For infection with adenoviruses, cells were infected overnight at a multiplicity of infection of 50 in serum-free medium.
Proliferation assays
For cell counting, cells were cultured in MCDB131 medium plus 2% fetal bovine serum (FBS). After 48 hours, cells were trypsinized and counted using a hemacytometer. For BrdU incorporation, cells were maintained in MCDB131 plus 2% FBS. After 24 hours, cells were pulsed with 10 μM of BrdU and incubated for an additional 24 hours. Cells were then fixed, labeled, and absorbance was measured at 370 nm using a BrdU Proliferation Assay (Roche, Indianapolis, IN).
RNA measurements
RNA was extracted with an RNeasy kit (Qiagen, Valencia, CA), and IL-8 mRNA transcript was detected using Northern blotting and a nonradioactive cDNA probe corresponding to bases 47 to 344 of human IL-8 mRNA. Expression of Ang-1 and Ang-2 mRNA in retrovirally transduced HUVECs was measured with TaqMan probes and a real-time polymerase chain reaction (PCR) apparatus (Applied Biosystems, Foster City, CA).
Immunoblotting and enzyme-linked immunosorbent assay
Total cell lysates, subcellular fractions, and immunoprecipitated complexes were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidenefluoride (PVDF) membranes, and then probed with monoclonal and polyclonal antibodies. Proteins were detected with horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescence (ECL) reagents. IL-8 protein in culture medium was detected with commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
IL-8 promoter activity
HUVECs were transfected with firefly luciferase reporter plasmid driven by the −133/+44 bp segment of human IL-8 promoter and 2 additional plasmids in which the AP-1 binding element (−126 to −120 bp) and NFκB-like factor binding element (−80 to −71 bp) have been mutated (Document S1).12 Cells were treated with either solvent or 300 ng/mL of Ang-1, and firefly luciferase activity was measured using the Dual Luciferase Assay kit and normalized to the relative renilla luciferase activity.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assays (EMSAs) for NFκB and activator protein-1 (AP-1) were performed with a Gelshift NFκB/Rel and AP-1 Family (TPA-inducible) kits (Active Motif, Carlsbad, CA) employing double-stranded DNA probes for human NFκB and human IL-8/AP-1 (Document S1) and 5 to 10 μg of nuclear extracts, according to the manufacturer's instructions.
Wound healing
Cell motility was assessed using the wound-healing assay as described previously.13 Murine stem-cell virus (MSCV) or MSCV-Ang1 cells were grown for 24 hours in basal medium. The media were collected and incubated at room temperature with anti–IL-8 neutralizing antibody (7.5 μg/mL) or IgG control antibody (7.5 μg/mL). Fresh MSCV and MSCV-Ang-1 were seeded into 24-well tissue culture plates and were then carefully wounded using a 200-μL pipette tip. Cellular debris was removed by washing, and the media were then replaced with the conditioned media of MSCV and MSCV-Ang-1 cells. After 12 hours, the wound healing was visualized with inverted bright field microscopy and quantified.
Migration assay
Migration of HUVECs was performed in 24-well transwell polycarbonate inserts (8.0 μm pore size) of modified Boyden chambers, as described.14 Conditioned media of MSCV or MSCV-Ang1 cells grown for 24 hours in basal medium were used as chemoattractants in the lower chamber. The apparatus was then incubated at 37°C in a CO2 incubator for 5 hours, to allow for cell migration, then quantified, as described.14
Statistics
Data were expressed as means plus or minus standard error (SE). Statistical significance was determined by one-way analysis of variance. Differences were considered statistically significant at P less than .05.
Results
Regulation of IL-8 mRNA and protein by Ang-1
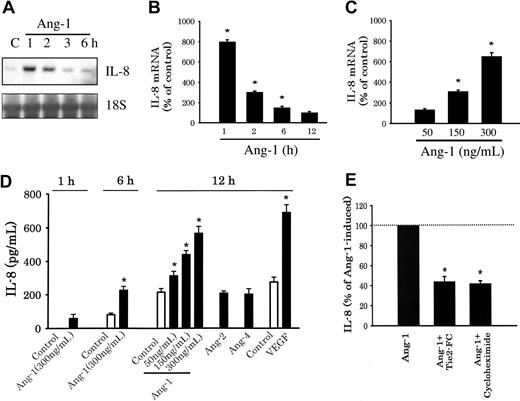
Ang-1 elicited significant, transient, and concentration-dependent increases in EC IL-8 mRNA levels (Figures 1A-C). In addition, Ang-1, but not Ang-2 or Ang-4, significantly induced IL-8 protein production in a time- and concentration-dependent fashion (Figure 1D). Similarly, vascular endothelial growth factor (VEGF) (80 ng/mL) induced a significant increase in IL-8 protein levels (Figure 1D). Ang-1–induced IL-8 protein was attenuated when HUVECs were preincubated with a soluble Tie-2 receptor protein (rhTie-2-FC) or the protein synthesis inhibitor cycloheximide (CHX; Figure 1E).
Ang-1 induces expression of IL-8 mRNA and protein. (A) A representative IL-8 Northern blot. HUVECs were serum starved for 12 hours and were then stimulated with Ang-1 (300 ng/mL) and collected 1, 2, 3, and 6 hours later. C indicates control samples, 18S refers to rRNA. (B) Means (± SEM; n = 3) of IL-8 mRNA intensity triggered by 300 ng/mL of Ang-1. (C) Means (± SEM; n = 3) of IL-8 mRNA intensity measured after 1 hour of Ang-1 addition. (D) Means (± SEM; n = 6) of IL-8 protein (detected with ELISA) in culture medium in response to Ang-1, Ang-2, and Ang-4 (300 ng/mL) and VEGF (80 ng/mL). (E) Means (± SEM; n = 6) of IL-8 protein measured after 12 hours of Ang-1 (100%) or Ang-1 plus rhTie2-FC protein (100× in excess of Ang-1) or Ang-1 plus cycloheximide (50 μg/mL, CHX). For panels C and D, *P < .05 compared with control values. For panel E, *P < .05 compared with Ang-1 alone.
Ang-1 induces expression of IL-8 mRNA and protein. (A) A representative IL-8 Northern blot. HUVECs were serum starved for 12 hours and were then stimulated with Ang-1 (300 ng/mL) and collected 1, 2, 3, and 6 hours later. C indicates control samples, 18S refers to rRNA. (B) Means (± SEM; n = 3) of IL-8 mRNA intensity triggered by 300 ng/mL of Ang-1. (C) Means (± SEM; n = 3) of IL-8 mRNA intensity measured after 1 hour of Ang-1 addition. (D) Means (± SEM; n = 6) of IL-8 protein (detected with ELISA) in culture medium in response to Ang-1, Ang-2, and Ang-4 (300 ng/mL) and VEGF (80 ng/mL). (E) Means (± SEM; n = 6) of IL-8 protein measured after 12 hours of Ang-1 (100%) or Ang-1 plus rhTie2-FC protein (100× in excess of Ang-1) or Ang-1 plus cycloheximide (50 μg/mL, CHX). For panels C and D, *P < .05 compared with control values. For panel E, *P < .05 compared with Ang-1 alone.
Generation of retrovirally transduced human umbilical vein endothelial cells
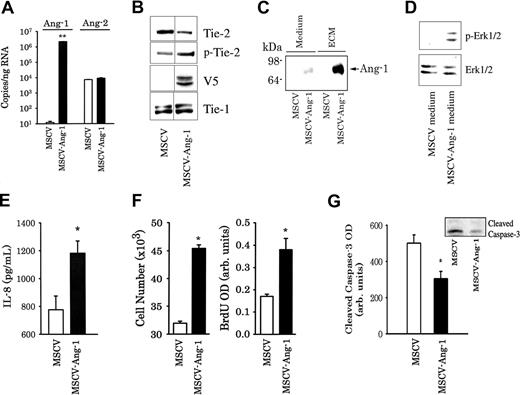
Previous studies have reported that Ang-1 elicits only a modest increase in EC proliferation. To augment the pro-proliferative properties of Ang-1 and to generate a cultured EC model that simulates the in vivo vasculature where Ang-1 is continuously being produced by vascular cells to stimulate EC Tie-2 receptors, we overexpressed Ang-1 in HUVECs, thereby allowing Ang-1 to strongly activate EC Tie-2 receptors in an autocrine fashion and stimulate EC proliferation. HUVECs were transduced with empty retroviruses (MSCV) or retroviruses expressing murine Ang-1 (MSCV-Ang-1). Real-time PCR confirmed the rise in murine Ang-1 mRNA expression in MSCV-Ang-1 cells (Figure 2A). Moreover, total Tie-2 protein levels decreased by 50%, and Tie-2 phosphorylation intensity increased by 90% in MSCV-Ang-1 compared with MSCV cells (Figure 2B). These results indicate strong activation of Tie-2 receptors in MSCV-Ang-1 cells. Ang-1 protein was detected mainly in the extracellular matrix (ECM) fraction of MSCV-Ang-1 cells, whereas relatively weaker Ang-1 protein levels were present in the media of these cells (Figure 2C). No Ang-1 protein was detected in the ECM and media of MSCV cells. Conditioned media of MSCV-Ang-1 cells, but not of MSCV cells, elicited a significant increase in Erk1/2 phosphorylation in control HUVECs (Figure 2D). In addition, media of MSCV-Ang-1 cells contained significantly greater IL-8 protein levels than that of MSCV cells (Figure 2E). Finally, cell number and BrdU incorporation over a 48-hour period were significantly greater, whereas cleaved caspase-3 intensities were significantly lower in MSCV-Ang-1 cells compared with MSCV cells (Figure 2F,G). We concluded that secreted Ang-1 protein in MSCV-Ang-1 cells is biologically active, is incorporated mainly in the ECM, and promotes IL-8 production, EC proliferation, and attenuates apoptosis.
Generation of HUVECs overexpressing murine Ang-1 using a retroviral vector. (A) Expression of murine Ang-1 and human Ang-2 mRNA levels in HUVECs transduced with control retroviruses (MSCV) and retroviruses expressing murine Ang-1 (MSCV-Ang-1). **P < .01 compared with MSCV cells. (B) Immunoblotting of MSCV and MSCV-Ang-1 cell lysates for Tie-2, tyrosine phosphorylated Tie-2 (p-Tie-2), V5 tag, and Tie-1 proteins. Vertical lines indicate that lanes were not directly adjacent to each other in the original blots. (C) Detection of Ang-1 protein in the medium and extracellular matrix (ECM) of MSCV and MSCV-Ang-1 cell using immunoblotting. (D) Erk1/2 phosphorylation measured after 15 minutes' exposure to conditioned medium of MSCV and MSCV-Ang-1 cells. HUVECs were cultured in complete medium overnight and were then serum starved for 6 hours. The medium was then removed and replaced by conditioned medium collected from serum-starved MSCV and MSCV-Ang-1 cells. (E) Detection of IL-8 protein in the medium of MSCV and MSCV-Ang-1 cells using ELISA (n = 6). *P < .05 compared with MSCV cells. (F,G) Means (± SEM; n = 6) of cell number, BrdU incorporation and cleaved caspase-3 intensity after 2 days' culturing in culture medium containing 2% FBS. *P < .05 compared with MSCV cells.
Generation of HUVECs overexpressing murine Ang-1 using a retroviral vector. (A) Expression of murine Ang-1 and human Ang-2 mRNA levels in HUVECs transduced with control retroviruses (MSCV) and retroviruses expressing murine Ang-1 (MSCV-Ang-1). **P < .01 compared with MSCV cells. (B) Immunoblotting of MSCV and MSCV-Ang-1 cell lysates for Tie-2, tyrosine phosphorylated Tie-2 (p-Tie-2), V5 tag, and Tie-1 proteins. Vertical lines indicate that lanes were not directly adjacent to each other in the original blots. (C) Detection of Ang-1 protein in the medium and extracellular matrix (ECM) of MSCV and MSCV-Ang-1 cell using immunoblotting. (D) Erk1/2 phosphorylation measured after 15 minutes' exposure to conditioned medium of MSCV and MSCV-Ang-1 cells. HUVECs were cultured in complete medium overnight and were then serum starved for 6 hours. The medium was then removed and replaced by conditioned medium collected from serum-starved MSCV and MSCV-Ang-1 cells. (E) Detection of IL-8 protein in the medium of MSCV and MSCV-Ang-1 cells using ELISA (n = 6). *P < .05 compared with MSCV cells. (F,G) Means (± SEM; n = 6) of cell number, BrdU incorporation and cleaved caspase-3 intensity after 2 days' culturing in culture medium containing 2% FBS. *P < .05 compared with MSCV cells.
Roles of IL-8 in migration and proliferation
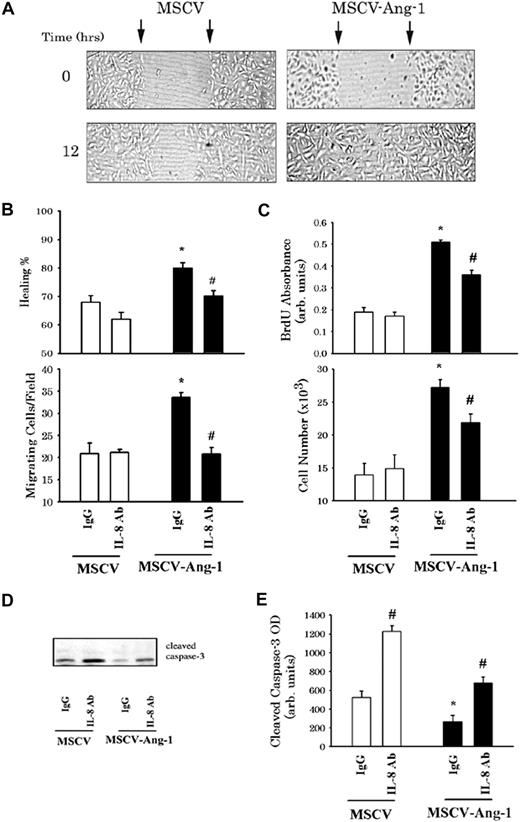
To evaluate the role of IL-8 in EC migration, we performed wound healing and the modified Boyden migration assays. For wound healing, MSCV and MSCV-Ang-1 cells (donor cells) were serum starved for 24 hours, and conditioned media were then collected and incubated with 7.5 μg/mL of a monoclonal anti–IL-8 antibody or an isotype control antibody (control IgG). This concentration of anti–IL-8 antibody was chosen based on successful blockade of IL-8 actions in ECs in previous studies.6 In addition, we verified in additional experiments that anti–IL-8 antibody (7.5 μg/mL) completely blocked the rise in HUVEC proliferation and migration measured in response to exogenous recombinant human IL-8 (Figure S1). Fresh MSCV and MSCV-Ang-1 cells were wounded with a pipette tip and cultured for 12 hours in IgG-neutralized conditioned media (Figure 3A). Wound healing was significantly more rapid in MSCV-Ang-1 cells compared with MSCV cells, when both cell types were maintained in control IgG-neutralized condition media (Figure 3B). The healing rate of MSCV-Ang-1 cells incubated with IL-8–neutralized condition media was significantly lower than that measured with control IgG-neutralized media. By comparison, neutralization of IL-8 in the conditioned media did not alter the healing rate in control (MSCV) cells (Figure 3B). Figure 3B shows the changes in HUVEC (wild-type) migration over a 5-hour period when conditioned media of MSCV or MSCV-Ang-1 cells were placed into the lower wells of modified Boyden chambers. Control IgG-neutralized conditioned media of MSCV-Ang-1 cells triggered significantly greater migration rates than did comparable conditioned media of MSCV cells (P < .05). Neutralization of IL-8 exerted no effect on the chemoattractive capacity of MSCV cell conditioned media, but significantly attenuated that of MSCV-Ang-1 cells (P < .05). These results suggest that IL-8 plays an important role in Ang-1–induced EC migration.
Ang-1–induced IL-8 plays a significant role in EC migration proliferation. (A) Representative examples of MSCV and MSCV-Ang-1 cell wounding experiments. MSCV (left panels) and MSCV-Ang-1 cells (right panel) were wounded (time 0) and maintained for 12 hours in conditioned media derived from MSCV and MSCV-Ang-1 cells, respectively, and were neutralized with IgG control antibody. Arrows point to the edges of the wounds. Note that wound healing (measured after 12 hours) was faster in MSCV-Ang-1 cells compared with MSCV cells. (B top panel) Mean (± SEM; n = 6) of wound healing in MSCV and MSCV-Ang-1 cells maintained for 12 hours in conditioned media of donor MSCV and MSCV-Ang-1 cells, respectively. MSCV or MSCV-Ang-1 cells were grown for 24 hours in basal medium. The media was collected and incubated with anti–IL-8 neutralizing antibody or IgG control antibody. Fresh MSCV and MSCV-Ang-1 cells were seeded into 24-well tissue culture plates and cultured in complete medium containing 20% FBS to nearly confluent cell monolayers. The cells were then carefully wounded using a pipette tip. After making the wounds, culture media were replaced with conditioned media of MSCV and MSCV-Ang-1 cells neutralized with anti–IL-8 or control IgG antibody. Wounds were then photographed (time = 0, and 12 hours later). Migration was evaluated by measuring the reduction in the diameter of the wound after migration of the cells into the cell-free zone. *P < .05 compared with MSCV cells maintained in IgG-neutralized conditioned media. #P < .05 compared with MSCV-Ang-1 maintained in IgG-neutralized conditioned medium. (B bottom panel) Means (± SEM; n = 6) of the number of HUVECs migrating toward conditioned media derived from MSCV or MSCV-Ang-1 cells. EC migration was performed in 24-well trans-well fibronectin-coated polycarbonate inserts. HUVECs were suspended in basal medium and seeded in the upper compartment. MSCV or MSCV-Ang-1 cells were grown in basal media for 24 hours, and conditioned media were then collected and incubated with anti–IL-8 and control IgG antibodies. Conditioned media were then placed into the lower compartment of the migration apparatus. Migration was quantified 5 hours later by counting cells in 10 fields per well. Symbols are the same as in panel B. (C) BrdU incorporation and cell number of MSCV and MSCV-Ang-1 cells maintained for 2 days in basal medium containing 2% FBS and IgG or anti–IL-8 antibodies. *P < .05 compared with MSCV cells maintained in the presence of IgG antibody. #P < .05 compared with MSCV-Ang-1 cells maintained in the presence of IgG antibody. (D,E) A representative immunoblot of cleaved caspase-3 and means (± SEM; n = 4) of cleaved caspase-3 intensity measured in MSCV and MSCV-Ang-1 cells after 2 days of culture in basal medium containing 2% FBS and IgG or anti–IL-8 antibodies. Symbols are the same as in panel B.
Ang-1–induced IL-8 plays a significant role in EC migration proliferation. (A) Representative examples of MSCV and MSCV-Ang-1 cell wounding experiments. MSCV (left panels) and MSCV-Ang-1 cells (right panel) were wounded (time 0) and maintained for 12 hours in conditioned media derived from MSCV and MSCV-Ang-1 cells, respectively, and were neutralized with IgG control antibody. Arrows point to the edges of the wounds. Note that wound healing (measured after 12 hours) was faster in MSCV-Ang-1 cells compared with MSCV cells. (B top panel) Mean (± SEM; n = 6) of wound healing in MSCV and MSCV-Ang-1 cells maintained for 12 hours in conditioned media of donor MSCV and MSCV-Ang-1 cells, respectively. MSCV or MSCV-Ang-1 cells were grown for 24 hours in basal medium. The media was collected and incubated with anti–IL-8 neutralizing antibody or IgG control antibody. Fresh MSCV and MSCV-Ang-1 cells were seeded into 24-well tissue culture plates and cultured in complete medium containing 20% FBS to nearly confluent cell monolayers. The cells were then carefully wounded using a pipette tip. After making the wounds, culture media were replaced with conditioned media of MSCV and MSCV-Ang-1 cells neutralized with anti–IL-8 or control IgG antibody. Wounds were then photographed (time = 0, and 12 hours later). Migration was evaluated by measuring the reduction in the diameter of the wound after migration of the cells into the cell-free zone. *P < .05 compared with MSCV cells maintained in IgG-neutralized conditioned media. #P < .05 compared with MSCV-Ang-1 maintained in IgG-neutralized conditioned medium. (B bottom panel) Means (± SEM; n = 6) of the number of HUVECs migrating toward conditioned media derived from MSCV or MSCV-Ang-1 cells. EC migration was performed in 24-well trans-well fibronectin-coated polycarbonate inserts. HUVECs were suspended in basal medium and seeded in the upper compartment. MSCV or MSCV-Ang-1 cells were grown in basal media for 24 hours, and conditioned media were then collected and incubated with anti–IL-8 and control IgG antibodies. Conditioned media were then placed into the lower compartment of the migration apparatus. Migration was quantified 5 hours later by counting cells in 10 fields per well. Symbols are the same as in panel B. (C) BrdU incorporation and cell number of MSCV and MSCV-Ang-1 cells maintained for 2 days in basal medium containing 2% FBS and IgG or anti–IL-8 antibodies. *P < .05 compared with MSCV cells maintained in the presence of IgG antibody. #P < .05 compared with MSCV-Ang-1 cells maintained in the presence of IgG antibody. (D,E) A representative immunoblot of cleaved caspase-3 and means (± SEM; n = 4) of cleaved caspase-3 intensity measured in MSCV and MSCV-Ang-1 cells after 2 days of culture in basal medium containing 2% FBS and IgG or anti–IL-8 antibodies. Symbols are the same as in panel B.
BrdU incorporation and cell number of MSCV-Ang-1 cells grown for 2 days in basal medium containing 2% FBS and control IgG antibody increased significantly, whereas the intensity of cleaved caspase-3 protein was significantly lower than in MSCV cells grown under similar conditions (Figure 3C-E). For a given cell type, neutralization of IL-8 with anti–IL-8 antibody resulted in a significant increase in the intensity of cleaved caspase-3 compared with IgG control antibody (Figure 3D,E). However, only in MSCV-Ang-1 cells did neutralization of IL-8 elicit a decline in BrdU incorporation and cell number (Figure 3C).
Contribution of the PI-3 kinase and MAPK pathways
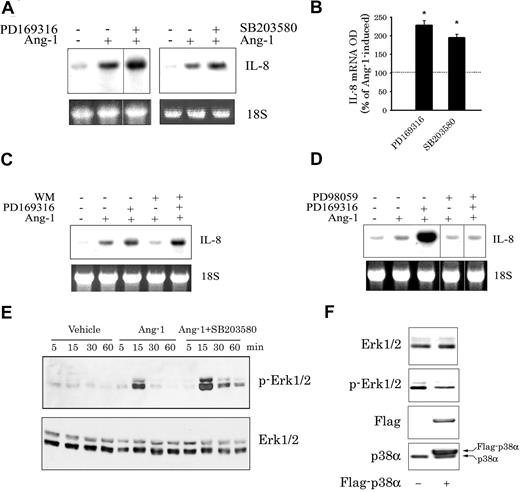
Ang-1 activates in ECs the PI-3 kinase and the Erk1/2, p38, and SAPK/JNK members of MAPKs.11,14 Preincubation with selective inhibitors of the Erk1/2 (PD98059 and U0126 both at 30 μM), the SAPK/JNK (15 μM SP600125), and the PI-3 kinase (50 nM wortmannin (WM) and 10 μM LY294002) pathways significantly attenuated Ang-1–induced IL-8 mRNA and protein levels (Figure 4). By comparison, preincubation with p38 pathway inhibitors (PD169316 and SB203580, both at 10 μM) augmented Ang-1–induced IL-8 expression (Figure 5A,B). This effect of p38 inhibitors was not altered by WM (Figure 5C), but was eliminated by PD98059 (Figure 5D). Thus, the rise in Ang-1–induced IL-8 mRNA expression by p38 inhibitors requires active Erk1/2, but not PI-3 kinase, pathway. These results also suggest that the p38 pathway inhibits Erk1/2 activation. This was confirmed by measuring the kinetics of Erk1/2 phosphorylation in response to Ang-1 in the absence and presence of SB203580. Figure 5E clearly indicates that Ang-1 exposure elicited a significant increase in Erk1/2 phosphorylation, which peaked after 15 minutes with a decline thereafter. However, when SB203580 (10 μM) was present in the culture medium, both the intensity and the duration of Erk1/2 phosphorylation elicited by Ang-1 were augmented. Moreover, overexpression of p38α protein in HUVECs elicited between 50% to 60% decline in Erk1/2 phosphorylation (Figure 5F). It should be noted that inhibition of Erk1/2 and p38 pathways had no effects on the degree of Ang-1–induced SAPK/JNK phosphorylation (Figure S2). Similarly, inhibition of the SAPK/JNK pathway with SP600125 didn't influence Ang-1–induced Erk1/2 and p38 activation (results not shown), suggesting that the negative cross-talk between the p38 and Erk1/2 pathways doesn't involve the SAPK/JNK pathway.
Roles of the PI-3 kinase and the SAPK/JNK and ERK1/2 MAPK pathways in Ang-1–induced IL-8 mRNA and protein expression. (A,B) Effects of wortmannin (WM, 50 nM), LY294002 (10 μM), SP600125 (15 μM), and PD98059 (30 μM) on IL-8 mRNA levels (representative blots in panel A and means [± SEM] in panel B) measured after 1 hour of Ang-1 exposure. Vertical lines in panel A indicate that lanes were not directly adjacent to each other in the original blots. (C) Mean (± SEM; n = 3) of IL-8 protein measured after 12 hours of Ang-1 (expressed as 100%) or Ang-1 plus U0126 (30 μM), PD98059 (30 μM), LY294002 (10 μM), or SP600125 (15 μM). *P < .05 compared with Ang-1 alone.
Roles of the PI-3 kinase and the SAPK/JNK and ERK1/2 MAPK pathways in Ang-1–induced IL-8 mRNA and protein expression. (A,B) Effects of wortmannin (WM, 50 nM), LY294002 (10 μM), SP600125 (15 μM), and PD98059 (30 μM) on IL-8 mRNA levels (representative blots in panel A and means [± SEM] in panel B) measured after 1 hour of Ang-1 exposure. Vertical lines in panel A indicate that lanes were not directly adjacent to each other in the original blots. (C) Mean (± SEM; n = 3) of IL-8 protein measured after 12 hours of Ang-1 (expressed as 100%) or Ang-1 plus U0126 (30 μM), PD98059 (30 μM), LY294002 (10 μM), or SP600125 (15 μM). *P < .05 compared with Ang-1 alone.
Role of the p38 MAPK in Ang-1–induced IL-8 expression. (A,B) Representative Northern blot and means (± SEM; n = 3) of IL-8 mRNA levels measured after 1 hour of Ang-1 (expressed as 100%) and Ang-1 plus PD169316 or SB203580 (both at 10 μM). *P < .05 compared with Ang-1 alone. (C) Representative Northern blot of IL-8 mRNA expression measured after 1 hour of Ang-1 and Ang-1 plus PD169316 (10 μM), WM (50 nM), or a combination of the 2. Vertical lines in panel A indicate that lanes were not directly adjacent to each other in the original blots. (D) Representative Northern blot of IL-8 mRNA expression measured after 1 hour of Ang-1 and Ang-1 plus PD169316 (10 μM), PD98059 (30 μM), or a combination of the 2. Vertical lines indicate that lanes were not directly adjacent to each other in the original blots. (E) Influence of p38 inhibition on Ang-1–induced Erk1/2 phosphorylation. Serum-starved HUVECs were incubated for 1 hour with SB203580 (10 μM) and were then stimulated with Ang-1 (300 ng/mL) for 5, 15, 30, and 60 minutes. Cells were then collected, and the levels of phosphorylation and total Erk1/2 proteins were detected with immunoblotting. Note the increase in both the intensity and duration of Erk1/2 phosphorylation when SB203580 was present in the medium. (F) Total and phosphorylated Erk1/2, Flag, and p38α proteins detected in HUVECs mock-transfected or transfected with Flag-p38α plasmid.
Role of the p38 MAPK in Ang-1–induced IL-8 expression. (A,B) Representative Northern blot and means (± SEM; n = 3) of IL-8 mRNA levels measured after 1 hour of Ang-1 (expressed as 100%) and Ang-1 plus PD169316 or SB203580 (both at 10 μM). *P < .05 compared with Ang-1 alone. (C) Representative Northern blot of IL-8 mRNA expression measured after 1 hour of Ang-1 and Ang-1 plus PD169316 (10 μM), WM (50 nM), or a combination of the 2. Vertical lines in panel A indicate that lanes were not directly adjacent to each other in the original blots. (D) Representative Northern blot of IL-8 mRNA expression measured after 1 hour of Ang-1 and Ang-1 plus PD169316 (10 μM), PD98059 (30 μM), or a combination of the 2. Vertical lines indicate that lanes were not directly adjacent to each other in the original blots. (E) Influence of p38 inhibition on Ang-1–induced Erk1/2 phosphorylation. Serum-starved HUVECs were incubated for 1 hour with SB203580 (10 μM) and were then stimulated with Ang-1 (300 ng/mL) for 5, 15, 30, and 60 minutes. Cells were then collected, and the levels of phosphorylation and total Erk1/2 proteins were detected with immunoblotting. Note the increase in both the intensity and duration of Erk1/2 phosphorylation when SB203580 was present in the medium. (F) Total and phosphorylated Erk1/2, Flag, and p38α proteins detected in HUVECs mock-transfected or transfected with Flag-p38α plasmid.
IL-8 mRNA stability
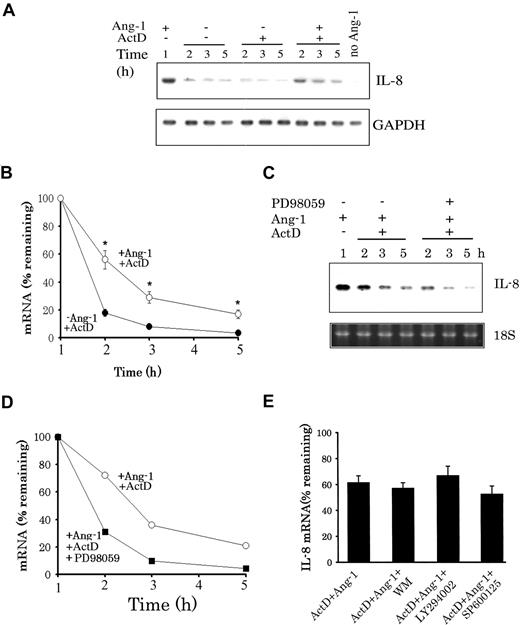
To evaluate the contribution of changes in mRNA stability to Ang-1–induced IL-8 mRNA expression, HUVECs were exposed to Ang-1 for 1 hour. Cells were then washed and maintained for 2, 3, and 5 hours in fresh medium or fresh medium containing ActD alone or ActD plus Ang-1. When Ang-1 and ActD were not present in the culture medium, IL-8 mRNA levels declined rapidly (Figure 6A). Similarly, low levels of IL-8 mRNA were observed when ActD was present in the culture medium. However, when Ang-1 was present along with ActD, IL-8 mRNA levels were significantly greater than those measured with ActD alone (Figure 6A,B), indicating that Ang-1 increases IL-8 mRNA stability. This effect of Ang-1 on IL-8 mRNA stability was eliminated when PD98059 (Erk1/2 inhibitor) was present along with Ang-1 (Figure 6C,D). By comparison, IL-8 mRNA levels in cells maintained in medium containing ActD plus Ang-1 plus WM, LY294002, or SP600125 were not different from those measured in cells exposed to ActD plus Ang-1 (Figure 6E). These results indicate that Ang-1 promotes IL-8 mRNA stability through activation of the Erk1/2 and that the PI-3 kinase and SAPK/JNK pathways are not involved in this response.
Ang-1 enhances IL-8 mRNA stability through Erk1/2 activation. (A) Cells were first exposed for 1 hour to Ang-1 and were then maintained in fresh medium, medium containing 5 μg/mL ActD (− Ang-1 + ActD), or ActD and Ang-1 (300 ng/mL; + Ang-1 + ActD). Cells were then collected after different time periods. Total RNA was then extracted, and IL-8 and glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA (controls) levels were detected with Northern blotting. (B) Means (± SEM; n = 3) of IL-8 mRNA intensities measured in cells undergoing protocols shown in panel A. mRNA intensities expressed as percentage of those measured after 1 hour of Ang-1 treatment. *P < .05 compared with −Ang-1 plus ActD. (C,D) Representative Northern blot and means (n = 3) of IL-8 mRNA. Cells were treated with Ang-1 for 1 hour and were then maintained in medium containing ActD plus Ang-1 (+ Ang-1 + ActD) and Ang-1 plus ActD + PD98059 (30 μM). Cells were then collected after different time periods, and total RNA was extracted and underwent Northern blotting for IL-8 and 18S levels. (E) Mean values (± SEM; n = 3) of IL-8 mRNA measured in cells which were treated first with Ang-1 for 1 hour (100% values) and were then maintained for an additional 1 hour in media containing Ang-1 plus ActD, Ang-1 plus AcD plus WM (50 nM), Ang-1 plus ActD plus LY294002 (10 μM), and Ang-1 plus ActD plus SP600125 (15 μM).
Ang-1 enhances IL-8 mRNA stability through Erk1/2 activation. (A) Cells were first exposed for 1 hour to Ang-1 and were then maintained in fresh medium, medium containing 5 μg/mL ActD (− Ang-1 + ActD), or ActD and Ang-1 (300 ng/mL; + Ang-1 + ActD). Cells were then collected after different time periods. Total RNA was then extracted, and IL-8 and glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA (controls) levels were detected with Northern blotting. (B) Means (± SEM; n = 3) of IL-8 mRNA intensities measured in cells undergoing protocols shown in panel A. mRNA intensities expressed as percentage of those measured after 1 hour of Ang-1 treatment. *P < .05 compared with −Ang-1 plus ActD. (C,D) Representative Northern blot and means (n = 3) of IL-8 mRNA. Cells were treated with Ang-1 for 1 hour and were then maintained in medium containing ActD plus Ang-1 (+ Ang-1 + ActD) and Ang-1 plus ActD + PD98059 (30 μM). Cells were then collected after different time periods, and total RNA was extracted and underwent Northern blotting for IL-8 and 18S levels. (E) Mean values (± SEM; n = 3) of IL-8 mRNA measured in cells which were treated first with Ang-1 for 1 hour (100% values) and were then maintained for an additional 1 hour in media containing Ang-1 plus ActD, Ang-1 plus AcD plus WM (50 nM), Ang-1 plus ActD plus LY294002 (10 μM), and Ang-1 plus ActD plus SP600125 (15 μM).
Roles of AP-1 and NFκB in Ang-1–induced IL-8 production
Ang-1 elicited a significant rise in the activity of a relatively short fragment of human IL-8 promoter (−133/+44 bp; Figure 7A). This effect was eliminated by inhibition of the Erk1/2, SAPK/JNK, and the PI-3 kinase pathways (Figure 7A). Furthermore, while mutation of the NFκB-like binding element (−80 to −71) had no effect on this response, Ang-1 failed to induce IL-8 promoter in which the AP-1 binding element (−126 to −120) was mutated (Figure 7A). Gel shift assays using oligonucleotides corresponding to AP-1 binding element of human IL-8 promoter revealed that Ang-1 significantly increased AP-1 DNA binding (225% ± 18%, Figure 7B). Supershift assays using antibodies specific for c-Jun and JunD impaired the formation or migration of AP-1/oligonucleotide complex both in vehicle- and Ang-1–treated cells, while antibodies for JunB, c-Fos, and FosB had no effect (Figure 7B). Moreover, Ang-1 treatment for 1 hour had no effect on mRNA expressions of Fos and Jun family members (Figure S3). Similarly, protein levels of c-Fos, phospho-Fos (Thr325), c-Jun, and JunD remained unchanged by Ang-1 treatment (Figure 7C). Phosphorylation of c-Jun on Ser63 and Ser73 are critical for transactivation of this protein and the rise in AP-1 DNA binding. Ang-1 induced a significant increase in c-Jun phosphorylation on Ser63 and Ser73 (Figure 7C). This effect was attenuated by Erk1/2 and SAPK/JNK inhibitors but not by PI-3 kinase inhibitors (Figure 7D). Thus, Ang-1 treatment triggered an increase in c-Jun phosphorylation and enhanced AP-1 DNA binding to IL-8 promoter. These responses were associated with increased IL-8 promoter activity and IL-8 protein production (Figure 8).
Roles of the transcription factors AP-1 in Ang-1–induced IL-8 production. (A) Top: structure of human IL-8 promoter (−133/+44) identifying AP-1 and NFκB-like binding elements. Sequences in brackets delineate in small letters mutations in these binding elements. Bottom: IL-8 promoter activity (normalized luciferase activity) measured in response to vehicle, Ang-1 and Ang-1 plus PD98059 (30 μM), WM (50 nM), and SP600125 (15 μM). *P < .05 compared with vehicle. Error bars represent SEM. (B) Binding of nuclear extracts from vehicle- and Ang-1–treated HUVECs to IL-8 specific AP-1 DNA probe in the absence (−) and presence of antibodies to c-Fos, FosB, c-Jun, JunB, and JunD. Competition with cold and mutated probes was performed as specificity control. (C) Effects of Ang-1 (300 ng/mL) on phosphorylation of c-Fos (Thr325), c-Jun (Ser63 and Ser73), total c-Fos, c-Jun, and JunD proteins. (D) The effect of 1 hour pretreatment with PD98059 (30 μM), SP600125 (15 μM), and WM (50 nM) on c-Jun phosphorylation (Ser63 and Ser73) measured after 15 minutes of Ang-1 (300 ng/mL) exposure.
Roles of the transcription factors AP-1 in Ang-1–induced IL-8 production. (A) Top: structure of human IL-8 promoter (−133/+44) identifying AP-1 and NFκB-like binding elements. Sequences in brackets delineate in small letters mutations in these binding elements. Bottom: IL-8 promoter activity (normalized luciferase activity) measured in response to vehicle, Ang-1 and Ang-1 plus PD98059 (30 μM), WM (50 nM), and SP600125 (15 μM). *P < .05 compared with vehicle. Error bars represent SEM. (B) Binding of nuclear extracts from vehicle- and Ang-1–treated HUVECs to IL-8 specific AP-1 DNA probe in the absence (−) and presence of antibodies to c-Fos, FosB, c-Jun, JunB, and JunD. Competition with cold and mutated probes was performed as specificity control. (C) Effects of Ang-1 (300 ng/mL) on phosphorylation of c-Fos (Thr325), c-Jun (Ser63 and Ser73), total c-Fos, c-Jun, and JunD proteins. (D) The effect of 1 hour pretreatment with PD98059 (30 μM), SP600125 (15 μM), and WM (50 nM) on c-Jun phosphorylation (Ser63 and Ser73) measured after 15 minutes of Ang-1 (300 ng/mL) exposure.
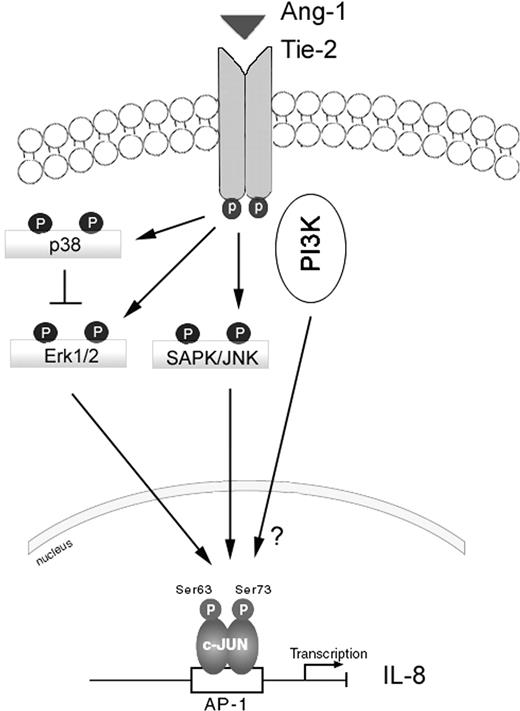
A schematic depicting the signaling pathways through which the Ang-1/Tie-2 receptor pathway regulates IL-8 production in HUVECs. Exposure to Ang-1 activates Tie-2 receptors which, in turn, provoke activation of the p38, Erk1/2, SAPK/JNK, and the PI-3 kinase pathways. While the Erk1/2 and SAPK/JNK pathways promote c-Jun phosphorylation and AP-1 activation leading to IL-8 induction, the mechanisms through which the PI-3 kinase pathway modulates IL-8 induction in response to Ang-1 are unclear, since this pathway didn't influence c-Jun phosphorylation. Also illustrated is the negative effect of the p38 MAPK pathway on Erk1/2 phosphorylation.
A schematic depicting the signaling pathways through which the Ang-1/Tie-2 receptor pathway regulates IL-8 production in HUVECs. Exposure to Ang-1 activates Tie-2 receptors which, in turn, provoke activation of the p38, Erk1/2, SAPK/JNK, and the PI-3 kinase pathways. While the Erk1/2 and SAPK/JNK pathways promote c-Jun phosphorylation and AP-1 activation leading to IL-8 induction, the mechanisms through which the PI-3 kinase pathway modulates IL-8 induction in response to Ang-1 are unclear, since this pathway didn't influence c-Jun phosphorylation. Also illustrated is the negative effect of the p38 MAPK pathway on Erk1/2 phosphorylation.
We excluded the involvement of NFκB in Ang-1–induced IL-8 production in HUVECs because Ang-1 had no effect on total IκB, phospho (Ser536) p65 levels and nuclear mobilization of the p65 and the p50 subunits of NFκB and had no influence on NFκB binding to DNA probe (Figure S4). Finally, transduction of HUVECs with adenoviruses expressing dominant negative forms of IKK-α or IKK-β failed to attenuate Ang-1–induced IL-8 expression (Figure S4).
Discussion
The main findings of this study are as follows: (1) Ang-1 induces significant increases in IL-8 production in HUVECs through induction of transcription and enhanced IL-8 mRNA stability; (2) the Erk1/2, SAPK/JNK, and PI-3 kinase pathways enhance IL-8 transcription in response to Ang-1, primarily through regulation of c-Jun phosphorylation and AP-1 binding to the IL-8 promoter, while increased IL-8 mRNA stability is mediated through the Erk1/2 pathway; (3) the p38 MAPK pathway exerts a negative influence on IL-8 production, a result of selective inhibition of Erk1/2 pathway activation; and (4) autocrine IL-8 effects are required for Ang-1–induced EC wound healing, migration, and proliferation.
We report here, for the first time, that Ang-1 induces the production of IL-8 in ECs, which acts in an autocrine fashion to enhance migration and proliferation of these cells. IL-8 is secreted by a variety of cells, including ECs, in response to cytokines, viruses, and oxidants. Previous studies have revealed that Ang-2 has no effect on IL-8 production in myelogenous leukemia blasts.15 By comparison, VEGF provokes a rapid induction of IL-8 mRNA in ECs, followed by a sustained increase in IL-8 protein levels.16 We have confirmed that VEGF induces IL-8 production in HUVECs (Figure 1). In this respect, induction of IL-8 expression in HUVECs by Ang-1 is qualitatively similar to that triggered by VEGF.
In contrast to our finding of an increase in IL-8 production in ECs by Ang-1, Pizurki et al8 have recently reported that Ang-1 attenuates thrombin-induced IL-8 production in EA.hy926 cells (hybrid ECs).8 We speculate that the discrepancies between our study and that of Pizurki et al are related to the cellular context and the nature of transcription factors involved in the induction of IL-8. For instance, up-regulation of IL-8 production by Ang-1 was mediated mainly through AP-1 binding, with the Erk1/2, SAPK/JNK, and PI-3 kinase pathways being upstream regulators. In comparison, thrombin induces substantial levels of IL-8 through activation of the p38 MAPK pathway and NFκB transcription factor.17,18 Hughes et al19 have demonstrated that Ang-1 inhibits NFκB activation through selective interaction with ABIN-2 (a novel inhibitor of NFκB). This finding could explain the inhibitory effect of Ang-1 on thrombin-induced IL-8 production reported by Pizurki et al In addition, while the p38 MAKP pathway promotes IL-8 production when thrombin is present,18 it inhibits IL-8 production in the presence of Ang-1 alone (Figure 5). These results suggest that the Ang-1/Tie-2 pathway might exert 2 opposing effects on IL-8 production, depending on the cellular context and the molecular mechanisms involved in the induction of the IL-8 promoter.
IL-8 gene expression is regulated through transcriptional activation and enhanced mRNA stability.20 Both of these mechanisms were invoked by Ang-1. Increased IL-8 mRNA stability by Ang-1 is consistent with previous reports documenting that cytokines (IL-1β and TNF-α) and growth factors (IGF-II and VEGF) enhance IL-8 mRNA expression, in part through increased mRNA stability.21 With respect to IL-8 transcription, many reports have identified NFκB and AP-1 transcription factors as the main regulators of IL-8 promoter activity in response to a variety of stimuli. In the current study, we have confirmed that Ang-1 triggers a significant rise in the activity of a relatively short fragment of human IL-8 promoter and that mutation of the AP-1 binding element in this portion (−126 to −120) abrogated this response (Figure 5). The involvement of AP-1 in Ang-1–induced IL-8 production was further confirmed by using gel shift assays (Figure 7). We should point out that because the influence of Ang-1 treatment on the activity of the full human IL-8 promoter wasn't assessed in this study, one could not exclude the involvement of transcription factors other than AP-1 and other modulators of IL-8 transcription such as reactive oxygen species in the induction of IL-8 production by the Ang-1/Tie-2 receptor pathway in ECs.
AP-1 transcription factors are composed of Jun (c-Jun, JunB, and JunD) family homodimers, Jun/Fos (c-Fos, FosB, Fra1, and Fra2), or Jun/ATF2 heterodimers. Abundance and phosphorylation of transactivation domains of these proteins regulate AP-1 transcriptional activity. We found that c-Jun phosphorylation on Ser63 and Ser73 was significantly elevated by Ang-1 exposure. c-Jun consists of a C-terminal DNA-binding/leucine zipper domain and an N-terminal trans-activation domain. De-phosphorylation of serine and threonine residues in the C-terminal of c-Jun is necessary for DNA binding, while phosphorylation on Ser63 and Ser73 in the N-terminal promotes AP-1 transactivation.22 Our observations that c-Jun phosphorylation at Ser63 and Ser73 was enhanced in response to Ang-1, and that the AP-1 complex bound to IL-8 DNA consisted mainly of c-Jun, suggest that c-Jun plays an important role in Ang-1–induced IL-8 production. One pathway responsible for increased c-Jun phosphorylation downstream from Tie-2 receptor is the SAPK/JNK pathway, which directly phosphorylates c-Jun on Ser63 and Ser73.23 We also found that Ang-1 triggers c-Jun phosphorylation through the Erk1/2 pathway (Figure 7D). It should be noted that this response is not dependent on a cross-talk between Erk1/2 and SAPK/JNK pathways, as Erk1/2 pathway inhibition had no effect on Ang-1–induced SAPK/JNK phosphorylation (Figure S2). One likely mechanism through which the Erk1/2 proteins regulate c-Jun phosphorylation is a direct action in which c-Jun might serve as a substrate for Erk proteins, as recently documented in embryonic fibroblasts.24 Finally, we report here that Ang-1 promotes IL-8 production, in part through the PI-3 kinase pathway. The exact molecular mechanisms involved in this response remain unclear. The observation that inhibition of PI-3 kinase pathway by wortmannin had no effects on c-Jun phosphorylation suggests that this pathway regulates IL-8 transcription through transcription factors other than AP-1 (Figure 7). The nature of these factors remains to be determined. Another possibility is that the PI-3 kinase pathway promotes IL-8 production by activating the Erk1/2 pathway as described previously.11 It is also possible that the PI-3 kinase pathway may promote AP-1 activity and IL-8 induction through inactivation of negative regulators of c-Jun DNA binding activity. One such regulator is glycogen synthase kinase 3 (GSK3), which phosphorylates c-Jun at C-terminal sites and inhibits the binding activity of this protein.25 By phosphorylating and inactivating GSK3 through protein kinase B (AKT), the PI-3 kinase pathway might trigger an increase in c-Jun DNA binding activity, leading eventually to increased IL-8 transcription.
Unlike the Erk1/2, SAPK/JNK, and PI-3 kinase pathways, the p38 MAPK pathway inhibits Ang-1–induced IL-8 production, an effect that is mediated through selective attenuation of Erk1/2 pathway activation. This negative cross-talk between the p38 and Erk1/2 pathways is not unique to ECs nor to the Ang-1/Tie-2 receptor pathway.26 In the case of the Ang-1/Tie-2 receptor pathway, the inhibitory role of p38 MAPKs may function as a biological switch through which other stimuli are able to modulate the degree to which the Ang-1/Tie-2 receptor pathway induces IL-8 and, by extension, EC proliferation and migration.
IL-8 is a chemoattractant for neutrophils and T lymphocytes and has been associated with inflammatory cell infiltration in many disease states. In addition, many reports have described important roles for IL-8 in embryonic and adult vascular formation and in tumor angiogenesis.27 The importance of IL-8 in tumor growth is mediated through both increased recruitment of inflammatory cells to the site of the tumors and through direct effects of IL-8 on ECs.28 In cultured ECs, IL-8 elicits angiogenic activities manifested by increased proliferation, migration, and in vitro capillary tube formation.10 It also inhibits apoptosis and induces MMP-2 production through activation of both CXCR1 and CXCR2 receptors in HUVECs.10 In the present study, we assessed the role of IL-8 in Ang-1–induced migration by using conditioned media of MSCV and MSCV-Ang-1 cells neutralized with anti–IL-8 or control IgG antibodies. Conditioned media of MSCV-Ang-1 neutralized with control IgG antibody elicited significantly faster healing rates in wound assays and greater EC migration in Boyden chamber assays, as compared with comparable media of MSCV cells. This suggests that Ang-1 elicits the release of soluble factors from MSCV-Ang-1 cells that promote EC migration. That IL-8 is one of these factors is indicated by the observation that neutralization of IL-8 results in disappearance of the promigratory effects of MSCV-Ang-1 conditioned media (Figure 3B). The results shown in Figure 3C also indicate that, in addition to promoting migration, IL-8 contributes, in part, to the enhancement of proliferation in MSCV-Ang-1. The roles played by IL-8 in Ang-1–induced EC migration and proliferation, therefore, are consistent with previous reports documenting that IL-8 acts in an autocrine fashion to promote migration and proliferation in cultured ECs.10 We should emphasize that even after IL-8 neutralization, cell number and BrdU incorporation in MSCV-Ang-1 cells remain higher than it does in MSCV cells, suggesting that factors other than IL-8 also contribute to the pro-proliferative effects of Ang-1.
We also assessed the contribution of IL-8 to the antiapoptotic properties of Ang-1 by comparing cleaved caspase-3 intensities in MSCV and MSCV-Ang-1 cells. We observed a significant increase in cleaved caspase-3 intensity in both cell types when IL-8 was neutralized with a selective antibody (Figure 3D,E). These results are consistent with the notion that IL-8 functions as an autocrine regulator of EC survival through activation of CXCR1 and CXCR2 receptors located on these cells. Promotion of EC survival by IL-8 is believed to be through up-regulation of the antiapoptotic members of the Bcl-2 family of proteins, including Bcl-2 and Bcl-XL.29 That cleaved caspase-3 intensity after IL-8 neutralization in MSCV-Ang-1 cells remained lower than that in MSCV cells suggests that additional mechanisms, other than IL-8, contribute to the antiapoptotic effects of Ang-1 in MSCV-Ang-1 cells. As it has been well established that the Ang-1/Tie-2 receptor pathway regulates EC survival through several mechanisms, including induction of Survivin-1 protein, activation of the Erk1/2 and PI-3 kinase pathways, and inhibition of the release of mitochondrial activators of caspases (Smac),2 any or all of these mechanisms might have contributed to the relatively lower levels of cleaved caspase-3 intensities in MSCV-Ang-1 cells.
We should emphasize that the importance of IL-8 in the in vivo functions of the Ang-1/Tie-2 receptor pathway, particularly the critical role of this pathway in embryonic vascular development, remains to be investigated. Documenting this importance, particularly in developing embryos, may prove to be difficult because of the well-described redundancy and overlap in the biological effects of chemokines and their receptors. For instance, genetic deletion of CXCR2, the main receptor for the murine chemokine equivalent of IL-8, is associated with significant up-regulation of CXCR1,30 suggesting that the absence of major vascular defects in CXCR2−/− mice might have been prevented as a consequence of compensatory increase in CXCR1 expression.31
In summary, we report here, for the first time, that Ang-1 elicits significant IL-8 production in HUVECs as a result of increased IL-8 transcription and improved IL-8 mRNA stability. These effects are mediated through activation of the Erk1/2, SAPK/JNK, and PI-3 kinase pathways, with AP-1 being the downstream transcription factor. Finally, enhanced IL-8 production plays important roles in mediating Ang-1–induced EC migration and proliferation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dominique Mayaki and Luigi Franchi for their technical assistance.
This work was supported by grants from the Heart and Stroke Foundation of Quebec and Canadian Institute of Health Research.
National Institutes of Health
Authorship
Contribution: N.A.-M. designed and performed research, analyzed data, and wrote the paper; C.B.S. contributed new analytical tools and designed research; A.S.K. provided new analytical tools and contributed new reagents; S.M. performed research; J.A.D.B. provided new reagents and analytical tools; S.N.A.H. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no com-peting financial interests.
Correspondence: S. Hussain, Rm L3.05, 687 Pine Ave West, Montréal, Québec, Canada H3A 1A1; e-mail: sabah.hussain@muhc.mcgill.ca.

![Figure 4. Roles of the PI-3 kinase and the SAPK/JNK and ERK1/2 MAPK pathways in Ang-1–induced IL-8 mRNA and protein expression. (A,B) Effects of wortmannin (WM, 50 nM), LY294002 (10 μM), SP600125 (15 μM), and PD98059 (30 μM) on IL-8 mRNA levels (representative blots in panel A and means [± SEM] in panel B) measured after 1 hour of Ang-1 exposure. Vertical lines in panel A indicate that lanes were not directly adjacent to each other in the original blots. (C) Mean (± SEM; n = 3) of IL-8 protein measured after 12 hours of Ang-1 (expressed as 100%) or Ang-1 plus U0126 (30 μM), PD98059 (30 μM), LY294002 (10 μM), or SP600125 (15 μM). *P < .05 compared with Ang-1 alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-08-110338/6/m_zh80080818040004.jpeg?Expires=1769149838&Signature=dRzYIjwin16jEZzP7GDqqos5dC5CN42F4Z-PrlNsDV7k3wbjrQxqZPo3tyhczbW6K4ZXRNBKE5uxZPU2nMDc4qfqf8xLAfZ0Vfd9~Mf9wjd7coyNfD2pvdtjvgubUGiiSFbaBtq~ocSRrBrNlPp3Za8TkIVgeVl~nvzP8gY2YnZ6Aiibdaa6EW7Msw0AcbTGyoBuY8BZp10145CF7UTNHa5-lj4koWO4abclZXbA6SEaY3jKwU42b7Vcs9xHJFUcNJtc8o5yRL2uEUsSR4Eu4wExf7OVZa6tPonSasXBysrKRBeffWhNvNWT6YOfG99vjpPuQiYp4S9U7rY1-HJu8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal