Abstract
Mutations in the adenosine deaminase (ADA) gene are responsible for a form of severe combined immunodeficiency (SCID) caused by the lymphotoxic accumulation of ADA substrates, adenosine and 2′-deoxy-adenosine. The molecular mechanisms underlying T-cell dysfunction in humans remain to be elucidated. Here, we show that CD4+ T cells from ADA-SCID patients have severely compromised TCR/CD28-driven proliferation and cytokine production, both at the transcriptional and protein levels. Such an impairment is associated with an intrinsically reduced ZAP-70 phosphorylation, Ca2+ flux, and ERK1/2 signaling and to defective transcriptional events linked to CREB and NF-κB. Moreover, exposure to 2′-deoxy-adenosine results in a stronger inhibition of T-cell activation, mediated by the aberrant A2A adenosine receptor signaling engagement and PKA hyperactivation, or in a direct apoptotic effect at higher doses. Conversely, in T cells isolated from patients after gene therapy with retrovirally transduced hematopoietic stem/progenitor cells, the biochemical events after TCR triggering occur properly, leading to restored effector functions and normal sensitivity to apoptosis. Overall, our findings provide a better understanding of the pathogenesis of the immune defects associated with an altered purine metabolism and confirm that ADA gene transfer is an efficacious treatment for ADA-SCID. The trials in this study are enrolled at www.ClinicalTrials.gov as #NCT00598481 and #NCT0059978.
Introduction
Adenosine deaminase (ADA) is a key enzyme in the purine pathway, ubiquitously expressed, catalyzing the irreversible deamination of adenosine (Ado) and 2′-deoxy-adenosine (dAdo). Genetic defects in ADA gene cause an accumulation of purine metabolites in plasma and cells, leading to an inherited form of severe combined immunodeficiency (SCID).1,2 Accordingly, broad lymphopenia, absence of humoral- and cellular-mediated immunity, recurrent infections, and failure to thrive are the prominent features of the most severe forms of ADA deficiency.3,4 In addition, the systemic accumulation of purine metabolites can cause alterations in several organs, including the skeleton, lung, liver, gastrointestinal tract, and central nervous system.4 The clinical phenotypes usually correlate with the severity of genetic mutations, the residual ADA activity and the extent of substrates accumulations.5
Conflicting data exist on the relative contribution of ADA metabolites in the pathogenesis of the immune dysfunctions. The biochemical hallmarks of ADA deficiency are consistent with the general belief indicating dAdo as the primary cause of lymphotoxicity in ADA-SCID. Exerting its effects at the nucleoside level or after conversion to dATP, accumulation of intracellular dAdo may interfere with deoxynucleotide synthesis and inhibit S-adenosylmethionine–mediated transmethylation reactions, which are required for cell viability and normal differentiation.6,7 In developing lymphocytes, dAdo has been reported to induce apoptosis through a pathway involving p53 and reversible by Bcl-2 overexpression.8,9 Interestingly, this mechanism does not seem to be implicated in the increased spontaneous apoptosis observed in mature T cells from ADA-SCID patients treated with enzyme replacement therapy.10 Alternatively, Ado may initiate an extracellular signaling by binding to G-protein coupled adenosine receptors present on the surface of target cells.11 Studies performed in the mouse model indicated that mature CD4+ and CD8+ T cells have decreased T-cell receptor (TCR)–triggered activation in vivo and in vitro as a result of exogenous Ado.12 Moreover, studies carried out with ADA-inhibitory drugs or selective A2A adenosine receptors agonists/antagonists showed that increased intracellular cAMP levels interfere with the proximal signaling events after TCR triggering, leading to inhibition of downstream effector functions.13-15 This evidence strongly suggests that the extracellular-related mechanisms of toxicity might be relevant in the pathophysiology of the disorder. Moreover, because of their cell-specific expression and regulation, aberrant adenosine receptor–mediated signaling may offer a better understanding of the autoimmune manifestations observed in some ADA-SCID patients.16,17 However, the molecular mechanisms linking the altered purine metabolism to the immunologic defect in humans remain to be fully elucidated.
The disease can be cured by bone marrow transplantation from an HLA-identical sibling donor18,19 or treated with lifelong injections of pegylated bovine ADA (PEG-ADA).20 Haploidentical bone marrow has also been reported to be successful, but the proportion of patients engrafting is lower than in other SCID types.21 Recently, we developed a successful gene therapy protocol, based on autologous transplantation of retrovirally transduced hematopoietic stem/progenitor cells (HSCs) combined with low-intensity conditioning, as an alternative therapeutic option.22 Results of this clinical trial showed the long-term correction of both metabolic and immunologic defects in ADA-SCID patients in the absence of PEG-ADA administration. Sustained gene marking was observed in multiple lineages, but the highest frequency of transduced cells was detected in T lymphocytes.22,23 These findings further confirm that T cells are particularly sensitive to an ADA-deficient environment, as ADA expression confers a strong selective advantage for their growth, differentiation, and function.
In the present work, we investigated the molecular mechanisms by which accumulating ADA substrates cause T-cell dysfunction. Here, we provide evidence that the immune defect in T cells from ADA-SCID patients is the result of an intrinsic inability to transduce activating signals through TCR, which is exacerbated by the suppressive effect of dAdo-mediated aberrant extracellular signaling. Importantly, these functional impairments are corrected in T cells isolated from patients after HSC gene therapy.
Methods
Patients and clinical trial
Patients were enrolled in ADA-SCID clinical trials approved by H. San Raffaele Scientific Institute and Hadassah Hospital Ethical Committees as well as by the national regulatory authorities. Informed consent was obtained from patients in accordance with the Declaration of Helsinki. Patients were diagnosed as ADA-SCID following early onset of typical viral, bacterial, and opportunistic infections at 1 to 5 months of age (Aiuti et al22 and A.A., B.C., C.B., M.G.R. et al, manuscript in preparation). Pt1 and Pt4 are homozygous for the same mutation, whereas Pt2, Pt3, and Pt5 are genetic compounds, carrying 2 different allelic mutations (A.A., B.C., C.B., M.G.R., manuscript in preparation). Five of the 7 mutations are known and already described as severe (class I, 0.015% ± 0.02% of wild-type activity) based on studies of recombinants in Escherichia coli.5 All patients displayed very low T-cell counts (< 0.05 × 109/L) and absent ADA activity in red blood cells24 (< 1% of normal). ADA activity in peripheral blood mononuclear cells, as measured before gene therapy, was detectable at low level in Pt1,22 whereas it was less than 2.5% of normal levels in Pt2 through Pt5.
Pt2, Pt3, and Pt4 received gene therapy after failure of haploidentical transplant, resulting in lack of detectable donor chimerism by polymerase chain reaction (PCR) analysis for HLA and STR (short tandem repeats) in peripheral blood T cells and bone marrow at the time of pre-GT evaluation. Pt5 was enrolled after low response to PEG-ADA and the pre-GT studies were performed while the patient was treated with enzyme replacement therapy. The patients received autologous BM-derived CD34+ cells prestimulated for 24 hours in the presence of cytokines (FLT3-ligand, stem-cell factor, thrombopoietin, interleukin-3 [IL-3]) and then transduced 3 times with the GIADAl MLV retroviral vector. Nonmyeloablative conditioning with 4 mg/kg busulphan intravenously (except Pt2, per os) total dose was administered on days −3 and −2 before CD34+ cell infusion, as previously described.22
In vitro expansion of T-cell lines
Blood samples from ADA-SCID patients and healthy donors were obtained after informed consent following standard ethical procedure and with approval of the San Raffaele Scientific Institute Ethical Committee for ADA-SCID GT (Gene ADA) and healthy donors. Untransformed T-cell lines were generated from peripheral blood mononuclear cells purified by density gradient centrifugation on Ficoll-Hypaque (Nycomed Pharma, Oslo, Norway). Cells were stimulated with 1 μg/mL phytohemagglutinin (Roche Diagnostics, Mannheim, Germany), 600 IU/mL rhIL-2 (Chiron Italia, Milan, Italy) in the presence of an allogeneic feeder mixture of 1 × 106/mL peripheral blood mononuclear cells (X-ray–irradiated at 60 Gy) and 105/mL JY cells25 (X-ray–irradiated at 100 Gy). The cultures were performed in Iscove modified Eagle medium (Lonza Milano, Milan, Italy) supplemented with 10% YSSEL medium (Dyaclone, Besançon, France), 5% Hyclone (Lonza Verviers SPRL), and 100 U/mL penicillin/streptomycin (Bristol-Myers Squibb, Rome, Italy). T cells were expanded in medium with rhIL-2 and restimulated as described every 14 days. CD4+ T cells were purified from established T-cell lines by immunomagnetic technique using anti-CD4 antibody-coated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions (purity was > 98%). All experiments shown were performed between days 12 and 15 of culture.
Reagents
Adenosine, 2′-deoxy-adenosine, the selective A2A adenosine receptor agonist (CGS21680), the selective A2A adenosine receptor antagonist (SCH58261), the nucleoside transporter inhibitor (dipyridamole), the adenylyl cyclase inhibitor (2′,5′-dideoxyadenosine 3′-triphosphate tetrasodium), and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma-Aldrich (St Louis, MO). The inhibitor of PKA (Rp-8-Br-cAMPS) was from Axxora (Nottingham, United Kingdom).
FACS staining
Anti-CD4, -CD8 mAbs directly coupled with fluorescein isothiocyanate (FITC) or PE and antiphospho-ZAP-70(Y319) Alexa 488 were from BD Biosciences (San Jose, CA). Apoptosis was assessed using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences). Cells were analyzed using a fluorescence-activated cell sorter (FACS) FACScan flow cytometer and CellQuest software (BD Biosciences).
DNA purification and quantitative PCR for vector-positive cells
Genomic DNA was extracted from T cells using QIAamp DNA Blood Mini kit (Qiagen, Alameda, CA). Quantitative PCR analysis for vector positivity was performed as described.26
Proliferation assay
CD4+ or bulk T-cell lines, starved overnight without IL-2, were cultured at 1 × 105/well in round-bottom 96-well plates precoated with the indicated dose of anti-CD3 mAb (OKT3, Janssen Cilag, Milan, Italy) with or without soluble anti-CD28 mAb (10 μg/mL; BD Biosciences). When indicated, the cells were stimulated in presence of drugs. After 48 hours, the cells were pulsed for 16 hours with 1 μCi/well [3H]thymidine (GE Healthcare, Little Chalfont, United Kingdom), then harvested and counted in a scintillation counter. Alternatively, starved T cells were incubated with carboxyfluorescein diacetate succinimidyl ester (2.5 μM, Invitrogen, Carlsbad, CA) in phosphate-buffered saline (PBS) and gently mixed for 10 minutes RT. Unbound carboxyfluorescein diacetate succinimidyl ester, or the deacetylated form, CFSE, was quenched by the addition of an equal volume of fetal bovine serum. The labeled cells were washed twice in complete medium and stimulated as previously described. At the time of harvest, CFSE-labeled cells were washed in PBS, and the dye TO-PRO-3 (Invitrogen) was added to each sample (1 nM final concentration) before acquisition to distinguish live and dead cells. Cells were harvested and analyzed 64 hours after CFSE labeling and 30 000 events were collected.
Cytokine detection
IL-2, IL-4, IL-5, IL-10, IFN-γ, and TNF-α proteins in cell culture supernatants were determined by capture ELISA according with the manufacturer's instructions (BD Bioscience). Intracellular cytokine production (IL-2, IL-4, and IFN-γ) was measured by intracytoplasmic FACS analysis of cells stimulated for 3 hours with either 1 μg/mL immobilized anti-CD3 mAb plus 10 μg/mL anti-CD28 mAb (BD Biosciences) or with 10 ng/mL TPA (12-otetradecanoylphorbol acetate) plus 500 ng/mL ionomycin. Cells were treated with 10 μg/mL Brefeldin A (Calbiochem, San Diego, CA) and incubated for additionally for 3 hours. Cells were fixed, permeabilized in blocking buffer (PBS 0.3% BSA, 0.1% NaN3), supplemented with 0.5% Saponin (Sigma-Aldrich), and subsequently stained with PE-labeled anti-IL-2, PE-labeled anti-IL-4, and FITC-labeled anti-IFN-γ (BD Biosciences).
To measure cytokine mRNA levels, CD4+ T-cell lines were stimulated with immobilized anti-CD3 mAb (1 μg/mL) plus soluble anti-CD28 mAb (10 μg/mL) for 5 hours. Total RNA was extracted from 1 × 106 cells using Eurozol reagent (Euroclone, Pero, Milan, Italy) and reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. IL-2, IL-4, IFN-γ, and hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA were quantitated using “assay on demand” real-time PCR kits (Applied Biosystems). The relative expression of cytokines was determined by calculating the difference between threshold cycles for the stimulated (target) and unstimulated (control) samples (ΔCT method) after normalization to HPRT, which was run in the same tube. The results were expressed as n-fold difference relative to the control sample.
Western blotting
CD4+ T cells from patients and controls were starved for 12 hours in low serum (1%) and then stimulated in the presence or not of dAdo (500 μM) by TCR cross-linking with 1 μg/mL anti-CD3 mAb plus 10 μg/mL anti-CD28 mAb for the indicated times. Cells were lysed in lysis buffer (1% SDS, 10 mM HEPES, and 2 mM EDTA pH 7.4) in the presence of inhibitors of proteases and phosphatases (Sigma-Aldrich), heated at 95°C for 5 minutes, and loaded (10 μg per lane) on 10% SDS-PAGE gels. On blotting onto nitrocellulose filters and blocking in TBS with 0.05% Tween-20 plus 5% nonfat dried milk, membranes were probed with antiphospho-p44/42 mAb and with antiphospho-cAMP response element-binding protein (CREB) mAb (Cell Signaling Technology, Danvers, MA), revealed by HRP system. Anti-p44/42 pAb, antitotal-CREB pAb (Cell Signaling Technology) and anti-GAPDH (Chemicon International, Temecula, CA) were used for protein normalization and control of loading. For all the experiments, the densitometric analysis was carried out with the ImageQuant software.
Intracellular cAMP determination
Bulk or CD4+ T cells (2 × 105 cells/well) were treated with dAdo or CGS21680, with or without the inhibitor of adenylyl cyclase, and in presence of IBMX. After 15 minutes of incubation, supernatants were discarded and cells used directly for cAMP determination, according to the manufacturer's protocol for the Direct cAMP Enzyme Immunoassay Kit (GE Healthcare).
Statistics
A 2-tailed Mann Whitney U test was used for the analysis of statistically significant differences comparing 2 independent groups of individuals, ADA-SCID patients vs healthy controls. Responses within the same individual were compared by the (2-tailed) Wilcoxon signed rank test for paired data.
Results
Characterization of the defective T-cell functions in ADA−/− CD4+ T cells
We established untransformed T-cell lines from 5 patients with severe ADA-SCID phenotype before (ADA−/−) and 6 to 48 months after HSC gene therapy (GT).22 The percentage of vector-positive cells by PCR ranged between 80% and 100% in GT cell lines. ADA expression measured by FACS analyses and ADA enzymatic activity in GT cell lines was restored to intermediate levels compared with age-matched healthy controls (NDs) (Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article). A markedly reduced S-adenosylhomocysteine hydrolase (SAHH) activity was found in ADA−/− T cells compared with NDs (Figure S1C, P < .01), indicating that an intrinsic metabolic defect persists in ADA−/− cells when cultured ex vivo. A similar defect was also observed in vitro in T cells derived from Pt5 during enzyme replacement therapy. In contrast, SAHH activity was completely restored at normal level in GT cells (Figure S1C).
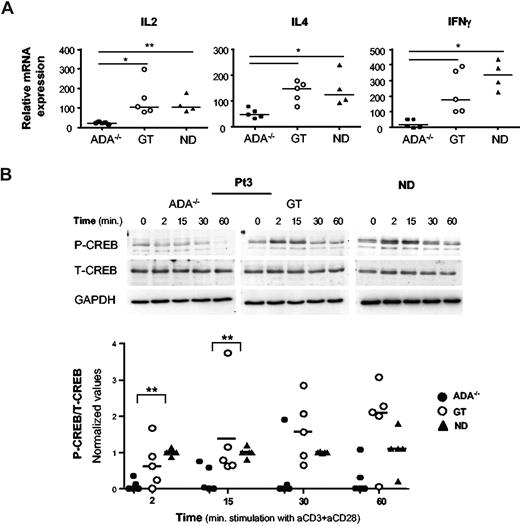
Next, we carried out a comprehensive functional characterization of CD4+ cells purified from patients' T-cell lines. Upon activation with escalating doses of plate-bound anti-CD3 mAb, the proliferative responses of ADA−/− CD4+ T cells were severely impaired, irrespective of the dose of mAb used (P < .01, Figure 1A). The addition of anti-CD28 mAb (10 μg/mL) enhanced the proliferative ability of ADA−/− T cells without achieving normalization (data not shown). In contrast, normal proliferative responses were observed in GT T-cell lines (Figure 1A). Secretion of IL-2, IFN-γ, TNF-α, IL-10 (P < .01, Figure 1B), and to a lesser extent IL-4 and IL-5 (P < .05, Figure 1B) was also compromised in ADA−/− T cells compared with normal cells. These results were confirmed when production of IL-2, IFN-γ, and IL-4 was measured at single-cell level. As shown in Figure 1C and Table 1, upon TCR/CD28 triggering, the percentages of IL-2 and/or IFN-γ-producing cells were strongly reduced in ADA−/− CD4+ cells with respect to NDs (P < .01). The production of IL-4 by ADA−/− CD4+ T cells was higher compared with other cytokines but still below the control range (P < .05; Figure 1C; Table 1). However, cells producing both IL-4 and IFN-γ were almost completely absent in ADA−/− cells. In gene-corrected CD4+ T cells, the production of both Th1- and Th2-like cytokines upon TCR stimulation was significantly improved compared with deficient cells (P < .05), reaching the normal range. Stimulation with TPA and calcium ionophore, which bypass the TCR, resulted in significantly higher production of all cytokines in ADA−/− T cells but confirmed the preferential production of Th2 cytokine (Figure 1C; Table 1). Collectively, these data indicate that in ADA−/− CD4+ T cells the cytokine production defect is TCR-dependent.
Characterization of the functional defect in CD4+ADA-deficient T cells. (A) Purified CD4+ T-cell lines from 5 patients and 5 NDs were stimulated with immobilized anti-CD3 mAb at the indicated concentrations, and [3H]thymidine incorporation was measured after 48 hours. One representative experiment of 3 is shown. (B) Cells were stimulated with 1 μg/mL anti-CD3, and culture supernatants were collected after 18 (IL-2) or 48 hours (all other cytokines). Cytokine concentrations were quantified by capture ELISA. Each value represents the mean of cytokine concentration measured in triplicate cultures for patients (n = 5; ADA−/− and GT) and healthy controls (n = 5). Median values are also reported. Background levels were subtracted. One determination of 3 is shown. (C) Production of IFN-γ vs either IL-2, or IL-4, was analyzed by intracytoplasmic staining in CD4+ T-cell lines as described in “Cytokine detection.” The experiment shown is representative of 5 independent experiments. Two ADA-SCID patients, before and after GT, and 2 NDs are reported. Range values corresponding to 5 patients and 7 NDs are indicated in Table 1.
Characterization of the functional defect in CD4+ADA-deficient T cells. (A) Purified CD4+ T-cell lines from 5 patients and 5 NDs were stimulated with immobilized anti-CD3 mAb at the indicated concentrations, and [3H]thymidine incorporation was measured after 48 hours. One representative experiment of 3 is shown. (B) Cells were stimulated with 1 μg/mL anti-CD3, and culture supernatants were collected after 18 (IL-2) or 48 hours (all other cytokines). Cytokine concentrations were quantified by capture ELISA. Each value represents the mean of cytokine concentration measured in triplicate cultures for patients (n = 5; ADA−/− and GT) and healthy controls (n = 5). Median values are also reported. Background levels were subtracted. One determination of 3 is shown. (C) Production of IFN-γ vs either IL-2, or IL-4, was analyzed by intracytoplasmic staining in CD4+ T-cell lines as described in “Cytokine detection.” The experiment shown is representative of 5 independent experiments. Two ADA-SCID patients, before and after GT, and 2 NDs are reported. Range values corresponding to 5 patients and 7 NDs are indicated in Table 1.
Range of cytokine producing cells from 5 patients (ADA−/− and GT) and 7 controls (ND) in 4 independent experiments
| . | aCD3/aCD28 . | TPA/ionomycin . | ||||
|---|---|---|---|---|---|---|
| IL-2+, cells (%) . | IL-4+, cells (%) . | IFN-γ+, cells (%) . | IL-2+, cells (%) . | IL-4+, cells (%) . | IFN-γ+, cells (%) . | |
| ADA−/− (n = 5) | 1.18-1.85* | 3.6-5.6** | 0.14-1.23* | 66.1-72.4 | 44.4-89.7 | 14.6-37.1* |
| GT (n = 5) | 7.4-24.8 | 10-29.2 | 2.8-23.2 | 63.5-89.4 | 33.1-67.4 | 14.3-59.2 |
| ND (n = 7) | 9.07-16.04 | 4.7-15.6 | 9.5-15.5 | 31.1-82.1 | 21.4-58.2 | 16.7-58.2 |
| . | aCD3/aCD28 . | TPA/ionomycin . | ||||
|---|---|---|---|---|---|---|
| IL-2+, cells (%) . | IL-4+, cells (%) . | IFN-γ+, cells (%) . | IL-2+, cells (%) . | IL-4+, cells (%) . | IFN-γ+, cells (%) . | |
| ADA−/− (n = 5) | 1.18-1.85* | 3.6-5.6** | 0.14-1.23* | 66.1-72.4 | 44.4-89.7 | 14.6-37.1* |
| GT (n = 5) | 7.4-24.8 | 10-29.2 | 2.8-23.2 | 63.5-89.4 | 33.1-67.4 | 14.3-59.2 |
| ND (n = 7) | 9.07-16.04 | 4.7-15.6 | 9.5-15.5 | 31.1-82.1 | 21.4-58.2 | 16.7-58.2 |
P < .01.
P < .05.
Defective cytokine transcriptional activity in ADA−/− CD4+ T cells
To investigate whether defective transcription of cytokine genes could account for their impaired production in ADA−/− cells, we analyzed by real-time PCR the cytokine mRNA levels in CD4+ T-cell lines. TCR/CD28-mediated activation induced significantly lower levels of IL-2, IL-4, and IFN-γ expression in ADA−/− T cells compared with NDs (Figure 2A; P < .01 for IL-2; P < .05 for IL-4 and IFN-γ) or post-GT cells (P < .05). Stimulation using TPA/ionomycin activated cytokine transcription similarly in ADA−/− and control CD4+ T cells (data not shown), indicating that the defect occurs at the transcriptional level only after TCR activation. Therefore, we examined the activation of 2 transcription factors, which are mandatory for the transcription of cytokine genes. Upon TCR engagement CREB is rapidly phosphorylated on Ser133, resulting in its activation.27 Patients and control CD4+ T cells were stimulated with cross-linked anti-CD3/CD28 mAbs, and CREB phosphorylation was analyzed by Western blot. As expected, Upon TCR triggering, CREB was rapidly (2 minutes) phosphorylated/activated in normal T cells, returning to basal level within 30 to 60 minutes of stimulation (Figure 2B). Remarkably, CREB activation was strongly impaired in ADA−/− cells from all 5 patients tested (Figure 2B, P < .01). In addition, CD3/CD28-induced phosphorylation of IkBα, which enables NF-kB to stably translocate to the nucleus,28 was profoundly inhibited in ADA−/− cells (P < .05, Figure S2). Conversely, pretranscriptional events involving CREB and IkBα phosphorylation were observed in GT cells, at variable extent, in agreement with the correction of the cytokine transcriptional defect (Figures 2, S2).
A defective transcription of cytokine genes in ADA-deficient T cells associates with reduced phosphorylation of CREB. (A) Cytokine gene expression after TCR/CD28-mediated stimulation of CD4+ T-cell lines from 5 patients (ADA−/− and GT) and 4 NDs. Total RNA was prepared from either resting or stimulated cells (1 μg/mL anti-CD3 plus 10 μg/mL anti-CD28 mAbs) and gene expression were measured by semiquantitative RT-PCR. Values were normalized for the expression of HPRT and correspond to duplicate samples. Results are expressed as n-fold difference relative to resting condition. One representative experiment of 3 is shown. **P < .01; *P < .05. (B) Immunoblots depicting phosphorylated CREB, total CREB, and GAPDH. CD4+ T cells were stimulated with cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti-CD28 mAbs for the indicated time points. The same membranes were sequentially probed for the different Abs. Upper panel: a representative experiment in Pt3 (ADA−/− and GT) and one ND is shown. Lower panel: densitometric analysis and statistical significance (**P < .01) on 5 patients (ADA−/− and GT) and 5 NDs are shown. Values corresponding to unstimulated cells were subtracted.
A defective transcription of cytokine genes in ADA-deficient T cells associates with reduced phosphorylation of CREB. (A) Cytokine gene expression after TCR/CD28-mediated stimulation of CD4+ T-cell lines from 5 patients (ADA−/− and GT) and 4 NDs. Total RNA was prepared from either resting or stimulated cells (1 μg/mL anti-CD3 plus 10 μg/mL anti-CD28 mAbs) and gene expression were measured by semiquantitative RT-PCR. Values were normalized for the expression of HPRT and correspond to duplicate samples. Results are expressed as n-fold difference relative to resting condition. One representative experiment of 3 is shown. **P < .01; *P < .05. (B) Immunoblots depicting phosphorylated CREB, total CREB, and GAPDH. CD4+ T cells were stimulated with cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti-CD28 mAbs for the indicated time points. The same membranes were sequentially probed for the different Abs. Upper panel: a representative experiment in Pt3 (ADA−/− and GT) and one ND is shown. Lower panel: densitometric analysis and statistical significance (**P < .01) on 5 patients (ADA−/− and GT) and 5 NDs are shown. Values corresponding to unstimulated cells were subtracted.
ADA−/− T cells have impaired proximal TCR signaling
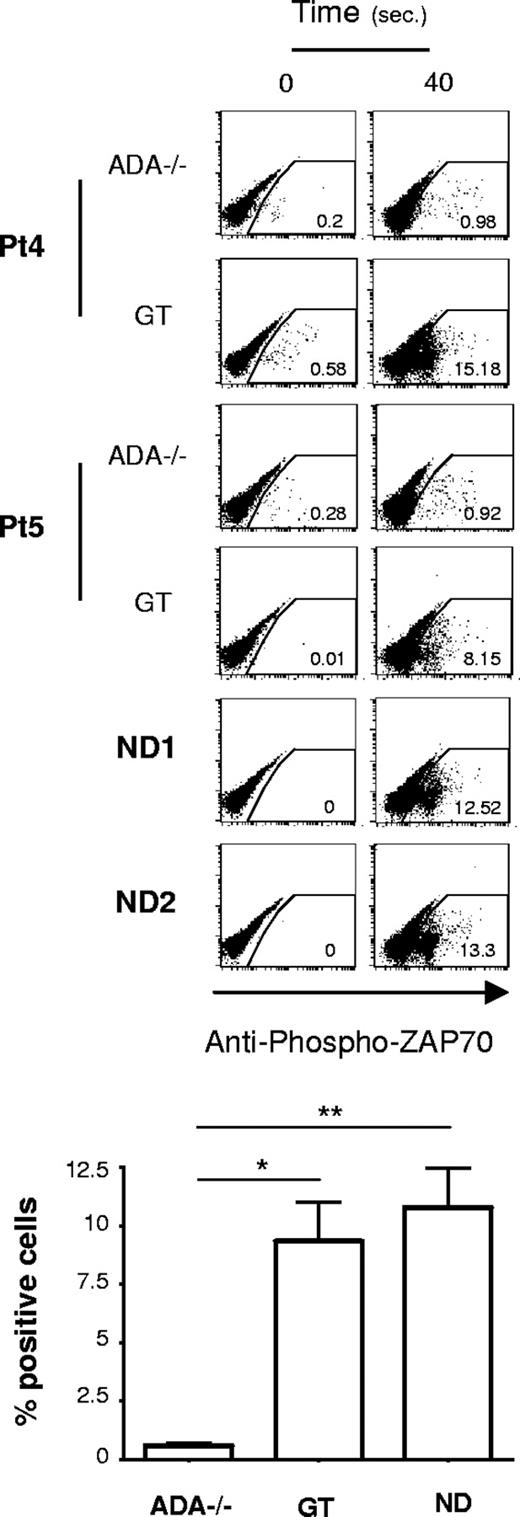
Given the early block in the transcriptional control, it was important to determine the step at which TCR signaling was impaired in ADA-SCID. To this aim, we analyzed very proximal T-cell signaling events. The phosphorylation of ZAP-70 (Y319) via cross-linking of the TCR/CD28 complex was evaluated by intracytoplasmic FACS analysis. As shown in Figure 3, NDs and GT T cells similarly displayed rapid ZAP-70 phosphorylation in response to TCR stimulation. Remarkably, in ADA−/− cells, this biochemical event takes place at a significantly lower extent (P < .01 vs NDs, P < .05 vs GT; Figure 3). In addition, analysis of the mean fluorescence intensity of the Ca2+-binding dye Fluo3 showed that the levels of Ca2+ were significantly reduced in ADA−/− T cells, after stimulation with anti-CD3, compared with controls (Figure S3).
Defective proximal TCR signaling in ADA-deficient T cells. Intracytoplasmic analysis of phosphorylated ZAP-70 (Y319) in response to cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti–CD28-mediated activation of CD4+ T cells. Dot plots from 2 ADA-SCID patients, before and after GT, and 2 NDs are reported. Results corresponding to frequency of positive cells after 40 seconds of stimulation, in one of 3 independent determinations, from 5 patients and 4 NDs are indicated in the graph. Values corresponding to basal phosphorylation were subtracted (*P < .05; **P < .01).
Defective proximal TCR signaling in ADA-deficient T cells. Intracytoplasmic analysis of phosphorylated ZAP-70 (Y319) in response to cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti–CD28-mediated activation of CD4+ T cells. Dot plots from 2 ADA-SCID patients, before and after GT, and 2 NDs are reported. Results corresponding to frequency of positive cells after 40 seconds of stimulation, in one of 3 independent determinations, from 5 patients and 4 NDs are indicated in the graph. Values corresponding to basal phosphorylation were subtracted (*P < .05; **P < .01).
dAdo-induced abrogation of T-cell activation in ADA−/− cells is mediated through A2A adenosine receptor
The most striking metabolic alteration in ADA-SCID is the massive accumulation of dATP in erythroid cells (range, 300-2000 μM)29 as the result of conversion of dAdo excreted in the body fluids. Thus, to further dissect the biochemical events linking an altered purine metabolism to the immune defect, we studied T-cell activation and effector functions in the presence of exogenous dAdo (500 μM). We first investigated the ERK1/2 signaling, the main pathway integrating TCR-activating signals in mature T cells. CD4+ T cells from patients and NDs were stimulated with cross-linked anti-CD3/CD28 mAbs in the absence or presence of dAdo, and activation of ERK1/2 pathway was examined by Western blot. In ADA−/− CD4+ T cells, ERK1/2 phosphorylation was consistently below the activation levels detected for the NDs, confirming that an intrinsic reduced T-cell activation underlies the functional defect observed in ADA−/− patient cells (Figure 4A and data not shown). Exposure to dAdo almost completely abolished ERK1/2 activation in ADA−/− CD4+ T cells, whereas in GT or ND T cells (n = 3) it resulted in delayed kinetics of activation (Figure 4A). These data provide clear evidence for an existing cross-talk between TCR- and dAdo-triggered biochemical pathways. Moreover, dAdo strongly inhibited TCR-driven proliferation of ADA−/− CD4+ cells, as measured by cell counts, CFSE dilution, and thymidine incorporation, whereas GT or ND T cells were only minimally affected (Figure 4B,C and data not shown). Because, at this dose, dAdo did not preferentially induce accumulation of TO-PRO-3 positive dying cells in culture (Figure 4B), these results show that dAdo can inhibit TCR-triggered activation independently from a direct cytotoxic effect.
dAdo strongly inhibits ERKs activation and effector functions in ADA-deficient T cells activating A2A AR signaling. (A) ERK activation was analyzed in CD4+ cells stimulated with cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti-CD28 mAbs in the absence or presence of dAdo (500 μM). Samples of untreated and dAdo-treated stimulated cells were prepared in parallel, run on different gels but probed with the different Abs at the same time. (B) CFSE-labeled CD4+ cells were cultured in plain medium or stimulated with immobilized anti-CD3 mAb (1 μg/mL) in the absence or presence of dAdo (500 μM). After 64 hours, cells were analyzed by flow cytometry. TO-PRO-3 was added at the time of FACS analysis. Histograms depicting the CFSE content of TO-PRO-3− viable cells are shown from one representative patient and one ND. The percentage of CFSEdim cells is indicated in each plot. Background levels corresponding to unstimulated cells were subtracted. The total number of trypan blue–negative viable cells and the frequency of TO-PRO-3–positive cells from CFSE/TO-PRO-3 dot plots of total events are shown for 5 patients and 4 NDs. (C) CD4+ cells stimulated with 1 μg/mL coated anti-CD3 mAb in the absence or presence of dAdo (500 μM) or (D) CGS21680 (100 μM). The specified compounds (SCH58261 or dipyridamole) were included 1 hour before the addition of dAdo. After 48 hours, cell proliferation was assessed by [3H]thymidine incorporation. The result of a representative experiment including 5 patients (ADA−/− and GT) and 5 NDs is reported. Percentages of inhibition with respect to the untreated condition are indicated. (E) Intracellular cAMP levels were determined in CD4+ T cells incubated for 15 minutes with culture medium containing 5 mM dAdo or 1 mM CGS21680. The inhibitor of adenylate cyclase (AC, 1 mM) was added 30 minutes before the other drugs. The data represent the mean plus or minus SD from 5 patients (ADA−/− and GT) and 4 NDs in one of 3 independent experiments. Statistics: **P < .01; *P < .05.
dAdo strongly inhibits ERKs activation and effector functions in ADA-deficient T cells activating A2A AR signaling. (A) ERK activation was analyzed in CD4+ cells stimulated with cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti-CD28 mAbs in the absence or presence of dAdo (500 μM). Samples of untreated and dAdo-treated stimulated cells were prepared in parallel, run on different gels but probed with the different Abs at the same time. (B) CFSE-labeled CD4+ cells were cultured in plain medium or stimulated with immobilized anti-CD3 mAb (1 μg/mL) in the absence or presence of dAdo (500 μM). After 64 hours, cells were analyzed by flow cytometry. TO-PRO-3 was added at the time of FACS analysis. Histograms depicting the CFSE content of TO-PRO-3− viable cells are shown from one representative patient and one ND. The percentage of CFSEdim cells is indicated in each plot. Background levels corresponding to unstimulated cells were subtracted. The total number of trypan blue–negative viable cells and the frequency of TO-PRO-3–positive cells from CFSE/TO-PRO-3 dot plots of total events are shown for 5 patients and 4 NDs. (C) CD4+ cells stimulated with 1 μg/mL coated anti-CD3 mAb in the absence or presence of dAdo (500 μM) or (D) CGS21680 (100 μM). The specified compounds (SCH58261 or dipyridamole) were included 1 hour before the addition of dAdo. After 48 hours, cell proliferation was assessed by [3H]thymidine incorporation. The result of a representative experiment including 5 patients (ADA−/− and GT) and 5 NDs is reported. Percentages of inhibition with respect to the untreated condition are indicated. (E) Intracellular cAMP levels were determined in CD4+ T cells incubated for 15 minutes with culture medium containing 5 mM dAdo or 1 mM CGS21680. The inhibitor of adenylate cyclase (AC, 1 mM) was added 30 minutes before the other drugs. The data represent the mean plus or minus SD from 5 patients (ADA−/− and GT) and 4 NDs in one of 3 independent experiments. Statistics: **P < .01; *P < .05.
To investigate the mechanism involved in the dAdo activity, we first examined T-cell proliferation in the presence of the A2A adenosine receptor (AR) selective antagonist SCH58261.30 Under these conditions, the suppressive effect of dAdo on ADA−/− T-cell proliferation was significantly reduced (P < .05, Figure 4C). Conversely, blocking of nucleoside uptake by dipyridamole had no protective effect and slightly enhanced the inhibitory properties of dAdo, in both patients and control cells (Figure 4C). This effect may probably rely on an increased dAdo extracellular availability, thereby amplifying its TCR-antagonizing activity. The selective A2A AR agonist, CGS21680,31 mimicked the suppressive effect of dAdo on cell proliferation, which, in turn, was blocked by SCH58261 (Figure 4D), thus supporting the idea that dAdo-related effects are initiated by an extracellular signaling rather than by its intracellular metabolism.
The Gs-protein coupled A2A adenosine receptor signals to increase intracellular cAMP.32 Measurement of cAMP levels in T cells cultured in presence of dAdo (5 mM) showed a strong increase over baseline in both patients and controls (Figure 4E). However, the intracellular cAMP levels were 2-fold higher in ADA−/− cells compared with normal and GT cells (P < .01 vs NDs; P < .05 vs GT) and not attributable to major differences in receptor density, as assessed at the protein and mRNA level (Figure S4). Furthermore, the A2A AR agonist induced comparable accumulation of cAMP in all cell types (Figure 4E). Altogether, these results indicate that dAdo abrogates T-cell function in ADA−/− cells via A2A AR and possibly by inducing altered level of intracellular cAMP.
PKA is the downstream effector of dAdo suppressive activity
The effects of cAMP in T cells are almost entirely mediated by cAMP-dependent protein kinase A type I (PKAI).33 To explore the potential involvement of PKA in the dAdo activity, we used a specific membrane-permeable inhibitor of cAMP-dependent PKA activation, Rp-8-Br-cAMPS.34 Addition of PKA inhibitor significantly reduced the suppressive effect exhibited by dAdo on proliferative responses of ADA−/− cells (P < .01, Figure 5A). No significant improvement in anti–CD3-induced proliferation was observed in ADA−/− T cells when Rp-8-Br-cAMPS alone was added to the cell culture (data not shown), suggesting that the intrinsic defect is not related to chronically elevated endogenous cAMP levels. Exposure to dAdo caused a broad inhibitory effect, even on cytokine production in T cells, with the most critical alterations in ADA−/− cells (P < .01 vs NDs, P < .05 vs GT; Figure 5B and data not shown). Again, the addition of PKA antagonist Rp-8-Br-cAMPS rescued the ability of ADA−/− T cells to produce cytokine on TCR stimulation (Figure 5B).
PKA hyperactivation is responsible for dAdo-mediated suppression of T-cell function in ADA-deficient cells. CD4+ T cells were stimulated with 1 μg/mL coated anti-CD3 mAb in the presence or absence of dAdo (500 μM) with or without the inhibitor of cAMP-dependent protein kinase A, Rp-8-Br-cAMPS (150 μM). (A) Rate of proliferation was evaluated by [3H]thymidine incorporation after 48 hours. (B) Culture supernatants were collected for cytokine production quantification by ELISA after 18 (IL-2) or 48 (all other cytokine) hours. Percentages of inhibition with respect to the untreated condition are indicated. The data represent the mean plus or minus SD of 5 patients (ADA−/− and GT) and 5 NDs from 1 of 3 independent experiments.
PKA hyperactivation is responsible for dAdo-mediated suppression of T-cell function in ADA-deficient cells. CD4+ T cells were stimulated with 1 μg/mL coated anti-CD3 mAb in the presence or absence of dAdo (500 μM) with or without the inhibitor of cAMP-dependent protein kinase A, Rp-8-Br-cAMPS (150 μM). (A) Rate of proliferation was evaluated by [3H]thymidine incorporation after 48 hours. (B) Culture supernatants were collected for cytokine production quantification by ELISA after 18 (IL-2) or 48 (all other cytokine) hours. Percentages of inhibition with respect to the untreated condition are indicated. The data represent the mean plus or minus SD of 5 patients (ADA−/− and GT) and 5 NDs from 1 of 3 independent experiments.
Increased susceptibility to apoptosis in ADA−/− cells on exposure to ADA substrates
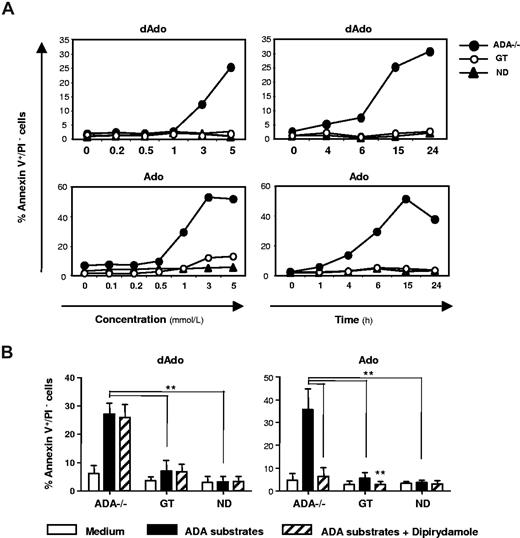
The metabolic consequences associated with ADA deficiency have been associated with apoptosis induction.8,9,35,36 To better define the role of apoptosis in the lymphocytopenia observed in patients, T cells were exposed to increasing concentrations of dAdo or Ado for different time, and apoptosis was assessed by annexinV/propidium iodide (PI) staining. A different kinetics in terms of time and concentration dependence was observed for ADA substrates, with Ado inducing apoptosis faster and at lower concentrations than dAdo in ADA−/− T cells (Figure 6A). Cumulative results from 5 patients showed that treatments with ADA metabolites induced a 3- to 4-fold higher proportion of apoptotic cells in ADA−/− T cells compared with normal cells (P < .01; Figure 6B). In contrast, GT cells were rescued from apoptosis similarly to NDs, in agreement with the expression of functional ADA (Figure 6B). Next, we evaluated the effects dAdo/Ado on cell viability in the presence of nucleoside transporter inhibitor. As shown in Figure 6B, dipyridamole was able to completely prevent Ado- but not dAdo-mediated apoptosis in ADA-deficient cells.
Dose- and time-dependent apoptosis induced in ADA-deficient T cells by ADA substrates. (A) Bulk T-cell lines were incubated with the indicated concentrations of purine metabolites and cultured for 15 hours (dAdo) or 6 hours (Ado). In time course experiments, T cells were incubated with dAdo (5 mM) or Ado (1 mM) for the indicated period. The percentages of early apoptotic cells (annexin V+/PI−) are shown for Pt3 (ADA−/− and GT) and one ND as representative experiment. (B) Bulk T-cell lines from 5 patients (ADA−/− and GT) and 6 NDs were cultured for 15 hours with dAdo (5 mM) or 6 hours with Ado (1 mM) in the absence or presence of the inhibitor of nucleoside transporter, dipyridamole (1 μM). The percentages of annexin V+/PI− cells as mean plus or minus SD are shown. Data are representative of at least 5 independent experiments (**P < .01).
Dose- and time-dependent apoptosis induced in ADA-deficient T cells by ADA substrates. (A) Bulk T-cell lines were incubated with the indicated concentrations of purine metabolites and cultured for 15 hours (dAdo) or 6 hours (Ado). In time course experiments, T cells were incubated with dAdo (5 mM) or Ado (1 mM) for the indicated period. The percentages of early apoptotic cells (annexin V+/PI−) are shown for Pt3 (ADA−/− and GT) and one ND as representative experiment. (B) Bulk T-cell lines from 5 patients (ADA−/− and GT) and 6 NDs were cultured for 15 hours with dAdo (5 mM) or 6 hours with Ado (1 mM) in the absence or presence of the inhibitor of nucleoside transporter, dipyridamole (1 μM). The percentages of annexin V+/PI− cells as mean plus or minus SD are shown. Data are representative of at least 5 independent experiments (**P < .01).
Discussion
In the present study, we uncover the molecular events underlying the immunologic defect of T cells from ADA-SCID patients and gain novel insights into the relative contribution of intracellular versus extracellular mechanisms of lymphotoxicity in the pathogenesis of this complex disease.
Impaired TCR/CD28-mediated proliferative responses, associated with a wide defect in cytokine production, were observed in ADA-deficient CD4+ T cells. The relative unbalance in Th2 versus Th1 cytokine production was observed at both the protein and mRNA levels. This bias might be related to the higher threshold of activation required for Th1 cytokine gene transcription with respect to Th2 gene because it has been reported that reduced ERK activation associates with early IL-4 production and Th2 differentiation.37 Analysis of T-bet and GATA-3 mRNA levels in resting and activated CD4+ T-cell lines revealed a significantly reduced expression of T-bet in ADA−/− compared with control cells (data not shown), thus arguing in favor of a Th2 skewing. In this view, our results are consistent with the clinical phenotype of ADA-SCID patients, characterized by recurrent allergic airway inflammations and markedly elevated IgE4,26,29 (A.A., B.C., C.B., M.G.R., manuscript in preparation). Moreover, the inability to produce IL-2 and IFN-γ by activated CD4+ cells and a general defect in Th1 differentiation probably account for a critical impairment in mounting protective cellular-mediated immunity in vivo, which might be responsible for the high incidence of opportunistic infections in these patients. Overall, the defect in cytokine secretion is only detected on TCR-mediated activation and is overcome by stimuli bypassing the TCR. Interestingly, normal cytokine production was observed after TPA/ionomycin stimulation of peripheral lymphocytes from patients treated with PEG-ADA,10 but the effects of TCR-mediated stimulation were not investigated.
The findings on cytokine genes transcription suggest that severely compromised immune functions in ADA-deficient T cells results from an inability to transduce activating signals from the membrane TCR/CD28 to the nucleus. Our observation that ZAP-70 phosphorylation and Ca2+ flux are generally defective in ADA−/− T cells complements and extends previous data obtained in thymocytes from ADA knockout mice.12 After TCR ligation, a rise in intracellular calcium levels sets the activation threshold and immunologic responsiveness through MAP-kinase pathway activation. Indeed, early ERK1/2 phosphorylation was found reduced in ADA-deficient cells.
Consistent with the impaired proximal TCR signaling, a reduction in the activation of CREB was observed early after TCR/CD28 triggering in ADA−/− T cells. Because CREB is involved in the expression of c-Jun, c-Fos, Fra-2, and FosB after TCR cross-linking,38 its functional absence might cause defective induction of AP-1 and IL-2 and subsequent proliferation impairment. In addition, TCR-dependent phosphorylation of IkBα was undetectable or markedly reduced in ADA-deficient cells, likely predicting decreased targeting to degradation39 and hence a low amount of NFkB translocated in the nucleus. Collectively, these data indicate that the immune defect in ADA-SCID involves multiple biochemical pathways, which are definitely required to elicit productive immune responses.
Interestingly, a reduced methylation of Vav-1 as well as an impaired activation of ERKs and of the nuclear transcriptional events linked to NFAT and NF-κB have been recently associated with drug-induced inhibition of SAHH enzyme.40 Reduction in SAHH activity, a classic hallmark of ADA-SCID,7,41,42 was observed in ADA−/− T cells but not in gene-corrected cells, suggesting that in these cells the transgene expression reaches a therapeutic level, enabling correction of both the metabolic and immunologic defects. Because normalization of SAHH activity is a sensitive indicator of normalized metabolite concentrations, these findings support the notion that an intracellular toxicity persists in ADA-deficient T cells, responsible for reduced TCR activation. The variable degree of functional correction observed after gene therapy may be explained by different levels of ADA expression at the single-cell level and/or by the presence of a residual fraction of untransduced cells.
To directly investigate the role of accumulating ADA substrates on the immunologic defect, we studied T-cell functions in the presence of exogenous dAdo. The most widely accepted dAdo-related pathogenetic mechanism in ADA-SCID is the intracellular poisoning derived from its nucleotide derives.43 Indeed, dAdo is a weaker agonist for adenosine receptors,44 and whether its local overload may be sufficient to initiate transmembrane signaling has not been carefully explored. We demonstrated that, at an adequate concentration, dAdo acts on the A2A AR inducing cAMP production and suppressing the activation of ADA-deficient T cells. The role of PKA in mediating the effects of cAMP in T cells has been described.45 It has been shown that the inhibitory properties of PKA were mediated via activation of Csk146 or inhibition of Raf1.47 Here we show that ADA-deficient T cells fail to phosphorylate ERKs when exposed to dAdo, indicating that ADA-deficient environment in vivo can directly antagonize biochemical events arising from TCR engagement. Because nuclear translocation of phosphorylated ERKs is required for cytokine production and cell- cycle progression,48-50 it is conceivable that dAdo-mediated inhibition of ERKs activation was responsible for the suppressed immune functions in ADA−/− T cells.
By evoking this state of lymphocyte unresponsiveness, dAdo might drive TCR-stimulated T cells toward an anergic rather than an immunogenic response. Indeed, anergic T cells are unable to activate lck, ZAP-70, phosphorylate TCR ζ chain, activate Ras and ERKs, and transactivate AP-1 and NF-AT.51-54 The cAMP/PKA pathway hyperactivation and SAHH inhibition may also play a key role in the induction and propagation of this anergic state, as they can affect mostly of these biochemical events, generally delivering off signals.33,40,55 Therefore, such a mechanism might be responsible for preventing T-cell activation and expansion during Ag-driven immune responses in vivo and may offer a comprehension for the clinical observations of autoimmunity in ADA-SCID patients with late-onset phenotype.16,17,29
The cause of T lymphopenia in ADA-SCID has been primarily attributed to induction of apoptosis by elevated levels of ADA metabolites, particularly in immature thymocytes,8,35,36,56 and consistently with the highest ADA expression in the thymus.4 In addition, a relatively high fraction of mature T lymphocytes isolated from ADA-SCID patients treated with PEG-ADA is committed to apoptosis,10 suggesting that this is a relevant mechanism of lymphopenia even in the peripheral blood of patients undergoing enzyme replacement therapy. In our study, we found that ADA-deficient T cells exhibit an increased susceptibility to apoptosis when exposed to high concentrations of ADA metabolites, confirming that, in addition to a general deregulation of T-cell function/homeostasis, a direct T-cell depletion is responsible for the complex picture of ADA-SCID clinical manifestation. Gene-corrected T cells were rescued from apoptosis, further demonstrating the efficacy of retroviral-mediated ADA gene transfer in restoring normal metabolic functions.
In conclusion, our study indicates that the activation-dependent defect induced by the intracellular toxicity and the active immunosuppression mediated by the extracellular adenosine receptor triggering evenly cooperate in controlling the immunologic defects and the clinical phenotype of ADA-SCID. In light of these results, the sole extracellular detoxification may not be sufficient to fully restore normal immune functions. Indeed, the majority of patients receiving enzyme replacement therapy do not achieve complete and sustained immune recovery,10,57,58 and T cells display in vitro functional and biochemical defects (Pt 5 and unpublished results). On the other hand, the correction of both the intracellular and extracellular mechanisms of disease observed in transduced T cells represents an important achievement, supporting the use of gene therapy as curative treatment for the immune disorder. It remains to be established whether ADA expressed by circulating or resident gene-corrected hematopoietic cells will be sufficient to correct long-term the nonimmune manifestations of the disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all physician and nurses of the Pediatric Clinical Research Unit of HSR-TIGET, Dr S. Slavin and Dr M. Aker at the Hadassah University Hospital for specimens of Pt1, and Dr Anna Mondino, Prof Mary Collins, and Dr Silvia Gregori for discussions.
This work was supported by grants from Italian Telethon Foundation, AFM/Telethon (GAT0205), Associazione Italiana per la Ricerca sul Cancro, and the European Community (project CONSERT LSBH-CT-2004-005242) as well as National Institutes of Health Grant 20 902 (M.H.) and a grant from Enzon Pharmaceuticals (M.H.).
National Institutes of Health
Authorship
Contribution: B.C. designed and performed research experiments, analyzed data, and wrote the paper; M.M. performed research experiments; F.C. and U.B. provided clinical care of the patients; M.H. contributed to analysis tools and revised the paper; F.C. and A.T. conducted the enzyme biochemical assays; C.B. contributed to the study design and revised the paper; M.G.R. supervised the research and revised the paper; A.A. supervised the research and wrote the paper; all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandro Aiuti, San Raffaele Telethon Institute for Gene Therapy, Via Olgettina 58, 20132, Milan, Italy; e-mail: a.aiuti@hsr.it.

![Figure 1. Characterization of the functional defect in CD4+ADA-deficient T cells. (A) Purified CD4+ T-cell lines from 5 patients and 5 NDs were stimulated with immobilized anti-CD3 mAb at the indicated concentrations, and [3H]thymidine incorporation was measured after 48 hours. One representative experiment of 3 is shown. (B) Cells were stimulated with 1 μg/mL anti-CD3, and culture supernatants were collected after 18 (IL-2) or 48 hours (all other cytokines). Cytokine concentrations were quantified by capture ELISA. Each value represents the mean of cytokine concentration measured in triplicate cultures for patients (n = 5; ADA−/− and GT) and healthy controls (n = 5). Median values are also reported. Background levels were subtracted. One determination of 3 is shown. (C) Production of IFN-γ vs either IL-2, or IL-4, was analyzed by intracytoplasmic staining in CD4+ T-cell lines as described in “Cytokine detection.” The experiment shown is representative of 5 independent experiments. Two ADA-SCID patients, before and after GT, and 2 NDs are reported. Range values corresponding to 5 patients and 7 NDs are indicated in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-05-092429/6/m_zh80070817480001.jpeg?Expires=1767709280&Signature=tKrwIkPOm5jpHa6bVJK4PLebXNSDbr81XwSfYQEsYW~AzVoe8EWUIKrdxKpbdTufspzxC5~xEN9-p-M6SkkkzxcBSjRkvMXDu6UrEPVWjnPdm~13Q7M6p2RInC06jFws867ZasOePBKHjLiDt9zf10MGQBSMdbkZWOs6mh8EErNJscRyDi8YcG4TcqB~A5OmWZvOFfEy-0LOEQCy7-qCviXd15hCkBCIMyG1JyaLsaRnmT-fX7xRVyBSri4pL-05xzVzrGIMUV3AMqjME88RjidN0J3-yzfQQYRU1wwYGzHvPyYMOLC1PBiWqS53dP9u5DagcCXyWuD8wN6PWiOIIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. dAdo strongly inhibits ERKs activation and effector functions in ADA-deficient T cells activating A2A AR signaling. (A) ERK activation was analyzed in CD4+ cells stimulated with cross-linked (1 μg/mL), anti-CD3 plus (10 μg/mL), anti-CD28 mAbs in the absence or presence of dAdo (500 μM). Samples of untreated and dAdo-treated stimulated cells were prepared in parallel, run on different gels but probed with the different Abs at the same time. (B) CFSE-labeled CD4+ cells were cultured in plain medium or stimulated with immobilized anti-CD3 mAb (1 μg/mL) in the absence or presence of dAdo (500 μM). After 64 hours, cells were analyzed by flow cytometry. TO-PRO-3 was added at the time of FACS analysis. Histograms depicting the CFSE content of TO-PRO-3− viable cells are shown from one representative patient and one ND. The percentage of CFSEdim cells is indicated in each plot. Background levels corresponding to unstimulated cells were subtracted. The total number of trypan blue–negative viable cells and the frequency of TO-PRO-3–positive cells from CFSE/TO-PRO-3 dot plots of total events are shown for 5 patients and 4 NDs. (C) CD4+ cells stimulated with 1 μg/mL coated anti-CD3 mAb in the absence or presence of dAdo (500 μM) or (D) CGS21680 (100 μM). The specified compounds (SCH58261 or dipyridamole) were included 1 hour before the addition of dAdo. After 48 hours, cell proliferation was assessed by [3H]thymidine incorporation. The result of a representative experiment including 5 patients (ADA−/− and GT) and 5 NDs is reported. Percentages of inhibition with respect to the untreated condition are indicated. (E) Intracellular cAMP levels were determined in CD4+ T cells incubated for 15 minutes with culture medium containing 5 mM dAdo or 1 mM CGS21680. The inhibitor of adenylate cyclase (AC, 1 mM) was added 30 minutes before the other drugs. The data represent the mean plus or minus SD from 5 patients (ADA−/− and GT) and 4 NDs in one of 3 independent experiments. Statistics: **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-05-092429/6/m_zh80070817480004.jpeg?Expires=1767709280&Signature=dHjoB46kg8z1flglFedjuRGcnrgt9ysOqjWm0niZJpRkQvNrzyTw8trIG5Hnp~f7-dUUEjo7zWehSg8HboxBSvJeQAD9tADxzgtdDEO3fGIox55Sj4wvGg9xAve5Y22Ov2TTZJA1Z44SGUyH0jAM6MkYX89i~b7EfwQzBrPrXMeJKDdu6yjnZZCr~o4JJgqcMUQlPOzfXv9uvdjePgABgQ~ehn4rehhPo7wETXaYQnb5uJjk4k2mAck7HVszDAL5rebouQykBoX4ln7zhWkOFP7Y-xnDlqAjQ7cyRBstBFhF91cuYiLJZM2RCDYZWWsKhf5dp7rBb9sTx~4hwDs81w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. PKA hyperactivation is responsible for dAdo-mediated suppression of T-cell function in ADA-deficient cells. CD4+ T cells were stimulated with 1 μg/mL coated anti-CD3 mAb in the presence or absence of dAdo (500 μM) with or without the inhibitor of cAMP-dependent protein kinase A, Rp-8-Br-cAMPS (150 μM). (A) Rate of proliferation was evaluated by [3H]thymidine incorporation after 48 hours. (B) Culture supernatants were collected for cytokine production quantification by ELISA after 18 (IL-2) or 48 (all other cytokine) hours. Percentages of inhibition with respect to the untreated condition are indicated. The data represent the mean plus or minus SD of 5 patients (ADA−/− and GT) and 5 NDs from 1 of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-05-092429/6/m_zh80070817480005.jpeg?Expires=1767709280&Signature=21NlJECag4UKJGEe8C7MQu1LqH6cXqGh9eR88Kq9uQki8AhW1dnFKKfh6y~P0AkpEBaDkXBdPRVhH~-nudPfvUpvv8vuQNTn20adlwlVN-Ct96BNSR8amxVtiigGuDZD3YboV5Nrn1jSXrjL1GyAw2KzImd1AStlkiS5qfHh~XsypgRFxTXH6r07wlFkm9Nle6ERxj9~dwFba3UNyEeFswg5FLOjam0nsdmZQdMC8ivRyuRu-DYss7o1Gcg33K37fMj2rRvlpBN9C2mkk2DQZ0hmFbXVqFu6Sedhvzp~9d-qh9R7TskLN56i-WQQjXfwTetQuVN36EWW5IA07Mp3qw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal