Abstract
Although graft-versus-host disease (GVHD) is a life-threatening complication of hematopoietic stem-cell transplantation (HSCT), its current diagnosis depends mainly on clinical manifestations and invasive biopsies. Specific biomarkers for GVHD would facilitate early and accurate recognition of this grave condition. Using proteomics, we screened for plasma proteins specific for GVHD in a mouse model. One peak with 8972-Da molecular mass (m/z) retained a discriminatory value in 2 diagnostic groups (GVHD and normal controls) with increased expression in the disease and decreased expression during cyclosporin A treatment, and was barely detectable in syngeneic transplantation. Purification and mass analysis identified this molecule as CCL8, a member of a large chemokine family. In human samples, the serum concentration of CCL8 correlated closely with GVHD severity. All non-GVHD samples contained less than 48 pg/mL (mean ± SE: 22.5 ± 5.5 pg/mL, range: 12.6-48.0 pg/mL, n = 7). In sharp contrast, CCL8 was highly up-regulated in GVHD sera ranging from 52.0 to 333.6 pg/mL (mean ± SE: 165.0 ± 39.8 pg/mL, n = 7). Strikingly, 2 patients with severe fatal GVHD had extremely high levels of CCL8 (333.6 and 290.4 pg/mL. CCL8 is a promising specific serum marker for the early and accurate diagnosis of GVHD.
Introduction
Hematopoietic stem-cell transplantation (HSCT) can be curative for many hematologic, oncologic, metabolic, and immune disorders. However, despite 2 decades of great advances in posttransplantation immunosuppressive therapy, graft-versus-host disease (GVHD) remains a major life-threatening posttransplantation complication. Thirty to eighty percent of hematopoietic stem cell (HSC) transplant recipients develop GVHD, the risk depending on the degree of histoincompatibility between donor and recipient, the type of transplantation, the number of T cells in the graft, the underlying disease, and the immunosuppressive treatment.1 Currently the diagnosis of GVHD is mainly clinical, based on skin rash, hyperbilirubinemia, and diarrhea. A simple noninvasive means of early and more precise diagnosis of GVHD would facilitate its early treatment and improve the outcome of HSCT
Recent advances in proteomics have created new techniques to examine the global expression of proteins in biologic fluids and to identify novel biomarkers in disease or pathologic states. One such technique is surface enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS), a high-throughput and sensitive proteomic approach to segregate proteins from complex bodily fluids such as plasma and to generate comparative protein profiles. In SELDI, proteins from complex biologic samples bind selectively to chemically modified affinity surfaces on a ProteinChip (Ciphergen Biosystems, Fremont, CA), with nonspecifically bound impurities washed away. Captured proteins are then analyzed by TOF-MS, producing spectra of the molecular mass (m/z) and relative concentration (intensity) of each protein. Recently this technology has been successfully applied to the diagnosis of cancer and other diseases.2-5
Previous reports have described proteomic analysis of human biofluids in GVHD.6-8 However, in human clinical studies unavoidable experimental artifacts related to genetic and environmental factors may confound the discovery of novel biomarkers. This is especially true in patients undergoing HSCT, who have a wide variety of preexisting diseases, undergo diverse conditioning regimens and GVHD prophylaxis, and have other variables. Mouse models provide an invaluable experimental system for studying human GVHD pathogenesis. To reduce these confounding variables, we looked first for diagnostic biomarkers for GVHD in a mouse model. These experiments suggested several candidate proteins linked to GVHD. One peak with 8972 Da m/z clearly discriminated between GVHD mice and normal controls with increased expression in the disease. Furthermore, administration of cyclosporin A to overt GVHD mice decreased the plasma level of this peak. In syngeneic transplantation, the 8972-Da peak was barely detectable. Purification and mass spectrometric analysis identified this molecule as CCL8, a member of a large chemokine family derived from a lineage of macrophages and other cell types. In human studies, we examined the serum concentration of CCL8 in relation to GVHD severity. CCL8 proved to be highly up-regulated in GVHD serum and 2 patients with severe, eventually fatal, GVHD had extremely elevated serum levels of CCL8. Our data suggest that serum CCL8 might be a highly specific test for the early and accurate clinical diagnosis of GVHD.
Methods
Mice
Male C57BL/6(H-2b) mice and female BALB/c(H-2d) mice were obtained from Sankyo Labo Service (Tokyo, Japan) and were bred in the Institute of Animal Experiment at Sapporo Medical University. Mouse ages ranged between 7 and 12 weeks at the start of experiments. The Sapporo Medical University Animal Experimental Ethics Committee approved the studies.
Reagents
All reagents were purchased from SIGMA/ALDRICH (Tokyo, Japan) unless otherwise indicated.
Bone marrow transplantation
On the day of bone marrow transplantation (BMT), donor mice (C57BL/6 for allogeneic BMT; BALB/c for syngeneic BMT) were killed by cervical dislocation. Donor bone marrow cells were collected into Dulbecco modified Eagle medium with 2% fetal calf serum/1% penicillin-streptomycin by flushing the femurs and tibias, and a single-cell suspension was prepared. Cells were washed and resuspended with RPMI 1640 medium for intravenous injection through the caudal vein. BM inoculum consisted of 2 × 107 BM cells for allogeneic and syngeneic BMT. Recipient BALB/c mice were fed with acidified water for at least 7 days before BMT to prevent sepsis after lethal irradiation. Recipient mice were given 8.5 Gy total body irradiation at a rate of 0.34 Gy/min and injected with donor BM cells within 3 hours.
GVHD monitoring
Recipient mice were monitored daily for clinical manifestations of GVHD including weight loss, hunched posture, skin erythema, alopecia, and diarrhea.
Histopathologic analysis
Recipient mice were killed on day 7, day 14, day 21, or day 28 after transplantation. Skin, liver, and small intestine were removed, fixed in 10% buffered formalin, and stained with hematoxylin and eosin for microscopy. Histologic changes in representative organs considered compatible with GVHD were as follows: skin (mononuclear infiltration into the dermo-epidermal junction and injury to hair follicles or sebaceous glands); liver (periportal mononuclear infiltration and hepatocellular necrosis); and small intestine (crypt cell apoptosis and dilatation or flattening of the villi). Findings were scored and given an overall interpretation of positive (+), indefinite (±), or negative (−) for GVHD.9,10 Representative histologic findings are shown in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Treatment of GVHD with cyclosporin A
Cyclosporin A (CsA; Novartis Pharma AG, Wilmington, DE) was diluted to 1.67 mg/mL with 0.9% NaCl. CsA 20 mg/kg daily was administered intraperitoneally from day 8 through day 13 after transplantation.
Treatment of mice with lipopolysaccharide or poly(I:C)
BALB/c mice received a transplant of 2 × 107 syngeneic marrow cells. On day 7 after transplantation, mice were injected intraperitoneally with either 5 μg lipopolysaccharide (LPS, Escherichia coli) or 5 μg poly(I:C) (GE Healthcare BioSciences, Tokyo, Japan) plus 20 mg D-GalN.11 Four hours after injection, blood was collected. Doses of LPS or poly(I:C) were determined by preliminary experiments.
Mouse plasma samples
Plasma was sampled before BMT on day 0, and after BMT on days 7, 14, 21, and 28. Blood was obtained from the tail vein of living mice using a capillary tube coated with heparin and then centrifuged at 2400g for 7 minutes within 30 minutes after bleeding. Aliquots of plasma were stored at −80°C until assayed.
SELDI protein chip array analysis
We added 20 μL of a solution containing 9 M/L urea and 10 g/L CHAPS in 10 mM Tris-HCl, pH7.4, to 10 μL of each sample. The mixture was vortex-mixed at 4°C for 15 minutes and diluted 1 to 40 in Tris-HCl. Eight-spot immobilized metal-affinity capture arrays (IMAC-30) were activated with 50 mM/L CuSO4. Diluted samples (50 μL) were applied to each spot on the protein chip array and incubated for 1 hour on a shaker. After washing with the same Tris-HCl followed by a quick water rinse, 0.5 μL saturated sinapinic acid (SPA) was applied twice to each spot and allowed to air-dry. Mass/charge (m/z) spectra of proteins bound to the chelated metal (through tryptophan, cysteine, histidine, or phosphorylated amino acids) were generated in a Ciphergen Protein Biology System II Time-of-Flight mass spectrometer (PBS II; Ciphergen Biosystems). Data were collected by averaging 65 laser shots with a laser intensity of 200 and a detector sensitivity of 8.
Statistical analysis of SELDI-TOF mass spectra
All spectra were compiled and we performed preliminary data analysis using Ciphergen ProteinChip Software 3.2.0 (Ciphergen Biosystems). The m/z values less than 2.0 kDa, corresponding to the signal from the SPA matrix, were omitted. Raw data were normalized and aligned by Biomarker Wizard (Ciphergen Biosystems). A classification tree was developed with Biomarker Pattern's Software (BPS; Ciphergen Biosystems) as previously described.12-14 Briefly, classification trees split the data into 2 nodes, using one rule at a time in the form of a question. The splitting decisions in this case were based on the normalized intensity levels of peaks or clusters identified from the SELDI protein-expression profile. Each identified peak or cluster becomes a variable in the classification process. Splitting is continued until terminal nodes are reached, and further splitting gives no gain in data classification. Multiple classification trees were generated using this process, and the best performing tree was chosen.
Protein purification
The 3 most abundant plasma proteins (albumin, IgG, transferrin) in the pooled plasma were removed by immunodepletion chromatography (Multiple Affinity Removal Column MS-3, 4.6 mmID × 50 mm; Agilent, Wilmington, DE). Plasma (50 μL) was diluted 5-fold in Buffer-A (Agilent), then injected onto the immunodepletion column. The flow-through fractions were collected and further resolved by high-performance liquid chromatography (HPLC, PU-2089plus; JASCO Engineering, Tokyo, Japan). The separation column used in HPLC was an Inertsil Ph column (5 μm, 4.6 mmID × 150 mm; GL Sciences, Tokyo, Japan). The elution gradient profile was as follows: (1) elution solvent A: 2% ACN/0.1% TFA, solvent B: 80% ACN/ 0.1% TFA; (2) liner gradient: 0% to 100% for solvent B for 50 minutes; flow rate: 1.0 mL/min. Fractions were collected every 30 seconds and their composition was monitored by SELDI-TOF MS; 2 μL of each fraction was applied on an Au chip (Ciphergen Biosystems) and processed with SPA matrix as described above. HPLC fractions containing candidate markers were selected according to SELDI-TOF MS monitoring. The fractionated samples were lyophilized and dissolved in 200 μL solubilization buffer (7 M urea, 2 M thiourea, 50 mM DTT, 2% ampholine, 3% CHAPS, 1% Triton X100). After sonication, sample solutions were loaded onto an IPG gel strip (pH 3-11 NL, 11 cm long; GE Healthcare BioSciences, Piscataway, NJ), and the strip was rehydrated for 10 hours at 30 V. The first-dimensional separation by isoelectric focusing (IEF) was carried out using the IPGphor system (GE Healthcare BioSciences, Tokyo, Japan) at 20°C for a total of 12 kV/h. After IEF, the IPG strips were equilibrated for 15 minutes in 50 mM Tris-HCl (pH 8.8) containing 6 M urea, 2% sodium dodecyl sulfate (SDS), 30% glycerol, 0.002% bromophenol blue, and 1% dithiothreitol and then re-equilibrated for 15 minutes in the same buffer except 2.5% iodoacetamide replaced dithiothreitol. For the second-dimensional separation, SDS–polyacrylamide gel electrophoresis (PAGE) was performed on homemade 8% to 20% gradient polyacrylamide gels and electrophoresed with a constant current of 40 mA/gel. After 2-dimensional electrophoresis (2DE), proteins were visualized with a silver staining kit (EzStain; ATTO, Tokyo, Japan).
Protein identification
Gel spots were washed with 100% ACN and 100 mM NH4HCO3, vacuum-dried, and then incubated in 5 μL trypsin solution (12.5 ng/μL in 50 mM NH4HCO3, 5 mM CaCl2) for 16 hours at 37°C. The resultant peptides were extracted once with 20 μL of 20 mM NH4HCO3 and 3 times with 20 μL of 5% formic acid/50% ACN. The collected extracts were vacuum-dried to approximately 40 μL and then analyzed by nanoflow HPLC-ESI-MS/MS. HPLC was performed using a DiNa system (KYA Technology, Tokyo, Japan), and tryptic digest samples were separated on a HiQsilC18W-3 column (75 μmID × 50 mm; KYA Technology). Elution solvent A was 0.1% formic acid, while solvent B was 70% ACN/0.1% formic acid. The gradient separation was 0% to 50% solvent B over 40 minutes at a flow rate of 200 nL/min. The separated peptides were characterized using a QSTAR XL Q-TOF mass spectrometer (Applied Biosystems, Foster City, CA). Data were acquired in information-dependent acquisition mode using Analyst Applied Biosystems, and only multiple charged ions were chosen for MS/MS. Each cycle was composed of 1-s MS and 2-s MS/MS. The acquired mass spectral data were automatically processed and searched against the Swiss Prot database using MASCOT software (Matrix Science, London, United Kingdom) and ProteinPilot software (Applied Biosystems). The search parameters were as follows: MS accuracy: 0.2 Da; MS/MS accuracy for the MASCOT search: 0.2 Da.
SELDI immunoassay (immunoSELDI)
PS20 (preactivated surface) ProteinChip surface (Ciphergen Biosystems) is composed of epoxides that form stable covalent linkages with amino groups of biomolecules (such as Abs). Ab (0.1 μg) was added to each spot of a PS20 chip and incubated for 2 hours at room temperature. After blocking residual active sites with 5 μL 1 M ethanolamine (pH 8.0) for 30 minutes, the spots were washed 3 times with 0.5% Triton X100 in PBS and twice with PBS. Mouse plasma samples were diluted 1:75 and human sera 1:25 with PBS and incubated for 2 hours under gentle mixing at room temperature. Each spot was washed twice with 0.5% Triton X100 in PBS and twice with PBS. After a rapid wash with 5 mM HEPES, SPA matrix was added and MS analysis performed using a PBS II ProteinChip reader.
Case reports
Fourteen patients underwent HSCT between April 1993 and December 2005 in the Department of Pediatrics, Sapporo Medical University Hospital. The median age was 8.9 years (range: 1-20 years, Table 3). The grading of GVHD was based on that used by Przepiorka et al15 Detailed clinical features of the patients in Figure 6 were as follows.
Patient A.
A 5-year-old boy with Fanconi anemia received a cord blood stem-cell transplant from an unrelated donor. He received pretransplantation conditioning composed of 3 Gy total body irradiation (TBI) with 200 mg/m2 fludarabine (Flu) and 40 mg/kg cyclophosphamide (CY), and GVHD prophylaxis with CsA and mycofenolate mofetil. GVHD first developed on day 13 after HSCT with skin rash (stage 2, grade II GVHD), and on day 26 diarrhea increased (grade III GVHD). Methyl prednisolone (mPSL) was started on day 13 (Figure 6A).
Patient B.
A 10-year-old boy with chronic myelogenous leukemia received a bone marrow transplant from an unrelated donor. He received pretransplantation conditioning composed of 12 Gy TBI with 120 mg/kg CY, and GVHD prophylaxis with tacrolimus and methotrexate. GVHD developed on posttransplantation day 19 with skin rash (stage 2, grade I GVHD), and diarrhea increased on day 27 (stage 3, grade III GVHD). mPSL was started on day 19 (Figure 6B).
Patient C.
A 3-year-old girl with acute lymphoblastic leukemia received a bone marrow transplant from an HLA-matched sister. Her pretransplantation conditioning was composed of 12 Gy TBI and 120 mg/kg CY, and her GVHD prophylaxis was methotrexate. She had no evidence of GVHD throughout her course (Figure 6C).
Human sera
The Sapporo Medical University Ethics Committee approved human sera studies. All volunteers and patients provided written permission and for young children we obtained written permission from their parents in accordance with the Declaration of Helsinki. Sera were aliquoted and stored at −80°C until assayed.
Enzyme-linked immunosorbent assay (ELISA) for human CCL8
Seven independent samples from 7 patients without GVHD and 7 from 7 patients with GVHD (more than grade II) were assayed by ELISA in duplicate. After HSCT, sera were obtained at the time of clinical diagnosis of GVHD. The concentration of CCL8 was quantified using an ELISA kit for human CCL8.
Immunologic reagents
Polyclonal antibodies against mouse or human CCL8 were purchased from Peprotech (London, United Kingdom). The human CCL8 ELISA kit was purchased from RayBiotech (Norcross, GA) and the mouse IL-6 kit from R&D Systems (Minneapolis, MN). These were used according to the manufacturer's protocol. Plates were read with a plate reader (Multiskan JX; Thermo Labsystems, Helsinki, Finland) at 450 nm.
Statistical analysis
The results are expressed as the mean plus or minus SE. Statistical significance analyses were performed by either paired or unpaired t test as indicated in the figure legends. A significant difference was set at P less than .05. The Bonferroni correction for multiple comparisons was applied. The results were representative data from a set of experiments.
Results
GVHD model
Acute GVHD was induced in BALB/c mice by injection of BM grafts from C57BL/6. In these mice, clinical symptoms of acute GVHD, such as diarrhea and ruffled fur, became apparent within 7 days, and skin erythema and alopecia developed within 21 days after transplantation. For controls, an identical number of syngeneic grafts were transplanted. The recipient mice who received allogeneic marrow cells but not those given syngeneic cells had pathologic evidence of GVHD at all time points after transplantation (Table 1; Figure S1).
Histologic findings of recipient mice
| Group/days . | Liver . | Intestine . | Skin . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| + . | +/− . | − . | + . | +/− . | − . | + . | +/− . | − . | |
| Syngeneic* | |||||||||
| 7 | 0 | 1 | 3 | 0 | 0 | 4 | 0 | 0 | 4 |
| 14 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 1 | 3 |
| 21 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 4 |
| 28 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 4 |
| Allogeneic† | |||||||||
| 7 | 5 | 1 | 0 | 4 | 2 | 0 | 4 | 2 | 0 |
| 14 | 6 | 0 | 0 | 6 | 0 | 0 | 5 | 1 | 0 |
| 21 | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 0 | 0 |
| 28 | 4 | 2 | 0 | 5 | 1 | 0 | 5 | 1 | 0 |
| Group/days . | Liver . | Intestine . | Skin . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| + . | +/− . | − . | + . | +/− . | − . | + . | +/− . | − . | |
| Syngeneic* | |||||||||
| 7 | 0 | 1 | 3 | 0 | 0 | 4 | 0 | 0 | 4 |
| 14 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 1 | 3 |
| 21 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 4 |
| 28 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 | 4 |
| Allogeneic† | |||||||||
| 7 | 5 | 1 | 0 | 4 | 2 | 0 | 4 | 2 | 0 |
| 14 | 6 | 0 | 0 | 6 | 0 | 0 | 5 | 1 | 0 |
| 21 | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 0 | 0 |
| 28 | 4 | 2 | 0 | 5 | 1 | 0 | 5 | 1 | 0 |
Formalin-fixed paraffin-embedded sections were stained with hematoxylin and eosin, randomized, and read blindly by an experienced pathologist (Y.K.). Findings, as mentioned in “Histopathologic analysis,” were scored and given an overall interpretation of positive (+), indefinite (+/−), or negative (−) for GVHD.
Days indicates days after bone marrow transplantation; and no., number of mice analyzed.
n = 4.
n = 6.
Protein profiling by SELDI-TOF MS
The peak intensity values of 169 differentially expressed peaks identified in all samples from 2.0 to 200 kDa mass ranges were used for further analysis. Classification trees were created using all 169 peaks, and on analysis a single peak with average mass of 8972 Da that distinguished GVHD plasma from normal plasma was identified (Table 2; Figure S2).
Peaks detected by SELDI screening of mouse plasma
| Up-regulated in GVHD . | Down-regulated in GVHD . | ||
|---|---|---|---|
| m/z . | Fold* . | m/z . | Fold . |
| 2941 | 22.2 | 7487 | −6.6 |
| 3222 | 12.3 | 7590 | −7.1 |
| 4391 | 12.4 | 7917 | −8.5 |
| 5085 | 6.1 | 15148 | −5.5 |
| 8972† | 12.3 | 15349 | −6.5 |
| 9182 | 9.9 | 15799 | −5.5 |
| 10561 | 8.3 | 15905 | −5.5 |
| 11049 | 7.3 | 15989 | −6.8 |
| 11279 | 9.6 | 31841 | −6.2 |
| 12821 | 10.0 | — | — |
| Up-regulated in GVHD . | Down-regulated in GVHD . | ||
|---|---|---|---|
| m/z . | Fold* . | m/z . | Fold . |
| 2941 | 22.2 | 7487 | −6.6 |
| 3222 | 12.3 | 7590 | −7.1 |
| 4391 | 12.4 | 7917 | −8.5 |
| 5085 | 6.1 | 15148 | −5.5 |
| 8972† | 12.3 | 15349 | −6.5 |
| 9182 | 9.9 | 15799 | −5.5 |
| 10561 | 8.3 | 15905 | −5.5 |
| 11049 | 7.3 | 15989 | −6.8 |
| 11279 | 9.6 | 31841 | −6.2 |
| 12821 | 10.0 | — | — |
Change in relative intensity of each ion peak between GVHD and control plasma is shown.
8972-Da peak selected for further study.
Increase of 8972-Da peak in GVHD plasma
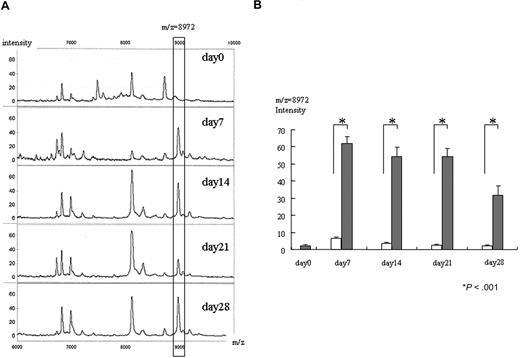
Figure 1A shows changes of intensity of the 8972-Da peak from an individual mouse at each time point. Compared with day 0 (before transplantation), we first detected an apparent increase of the 8972-Da peak on day 7 after BMT. The intensity of the 8972-Da peak was 4.6 at day 0, which increased to 47.3, 51.4, 25.2, and 55.9 on days 7, 14, 21, and 28, respectively. All increases in peak intensity of GVHD samples were significantly higher than those in syngeneic controls at posttransplantation sampling days 7, 14, 21, and 28 (Figure 1B). The increase on days 7, 14, 21, and 28 of allogeneic samples was also significantly higher than the day-0 samples.
Increase of 8972-Da peak in mouse GVHD plasma. (A) Representative SELDI spectra of the 8972-Da peak at day 0 obtained from pretransplantation and GVHD plasma (days 7, 14, 21, and 28 after transplantation) from an individual mouse are shown. The m/z range of 6000 to 10 000 m/z is shown. The box highlights an 8972-Da peak that is increased in intensity in GVHD plasma compared with day-0 samples. Number at the top and bottom of the figure indicates m/z. The left column indicates relative intensity of the ion peak. The Peak intensity of 8972 Da is 4.6, 47.3, 51.4, 25.2, and 55.9 on days 0, 7, 14, 21, and 28, respectively. (B) Mean normalized intensity values for 8972-Da peak in samples at each time point (day 0 is before transplantation; days 7, 14, 21, and 28 are after transplantation). The average intensity ± SE is depicted (n = 9). All GVHD samples (closed column except day-0 samples) were significantly higher than that of syngeneic controls (open column) on posttransplantation days 7, 14, 21, and 28 as judged by an unpaired t test. Increases on days 7, 14, 21, and 28 of allogeneic samples were also significantly higher than that of day-0 samples (before transplantation) (paired t test). Asterisk indicates P value.
Increase of 8972-Da peak in mouse GVHD plasma. (A) Representative SELDI spectra of the 8972-Da peak at day 0 obtained from pretransplantation and GVHD plasma (days 7, 14, 21, and 28 after transplantation) from an individual mouse are shown. The m/z range of 6000 to 10 000 m/z is shown. The box highlights an 8972-Da peak that is increased in intensity in GVHD plasma compared with day-0 samples. Number at the top and bottom of the figure indicates m/z. The left column indicates relative intensity of the ion peak. The Peak intensity of 8972 Da is 4.6, 47.3, 51.4, 25.2, and 55.9 on days 0, 7, 14, 21, and 28, respectively. (B) Mean normalized intensity values for 8972-Da peak in samples at each time point (day 0 is before transplantation; days 7, 14, 21, and 28 are after transplantation). The average intensity ± SE is depicted (n = 9). All GVHD samples (closed column except day-0 samples) were significantly higher than that of syngeneic controls (open column) on posttransplantation days 7, 14, 21, and 28 as judged by an unpaired t test. Increases on days 7, 14, 21, and 28 of allogeneic samples were also significantly higher than that of day-0 samples (before transplantation) (paired t test). Asterisk indicates P value.
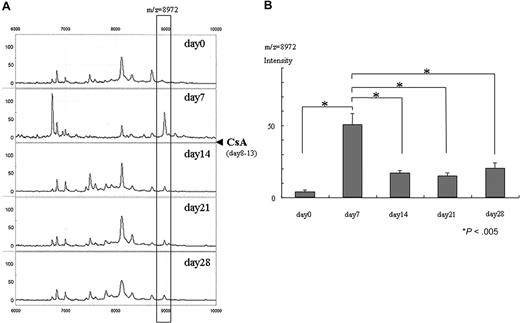
CsA treatment decreased intensity of the 8972-Da peak in GVHD mice
Figure 2 shows the effects of CsA treatment on the 8972-Da peak. Its intensity was 5.8 on day 0 and it increased to 69.4 on day 7. CsA was administered from day 8 to day 13, and the 8972-Da peak decreased to 13.8, 15.7, and 14.3 on days 14, 21, and 28, respectively (Figure 2A). This decrease was statistically significant (Figure 2B).
Decrease of 8972-Da peak after CsA administration in GVHD mice. (A) Representative SELDI spectra of control plasma (day 0; before transplantation) and GVHD plasma (days 7, 14, 21, and 28 after transplantation) from the same mouse. The m/z range of 6000 to 10 000 m/z is shown. CsA was administered from day 8 through day 13 after transplantation, indicated by closed triangle. The box highlights a plasma peak with an average mass of 8972 Da that decreased after administration of CsA. The peak intensity of 8972 Da is 5.8, 69.4, 13.8, 15.7, and 14.3 on days 0, 7, 14, 21, and 28, respectively. (B) Mean normalized intensity for the 8972-Da peak in the samples on days 0 (before transplantation), 7, 14, 21, and 28 (n = 6, respectively). The average intensity (± SE) is depicted (n = 6). The 8972-Da peak seen at day 7 significantly decreased at days 14, 21, and 28 (paired t test). The peak at day 7 is significantly higher than that of day 0 (before transplantation) (paired t test). Asterisk indicates P value.
Decrease of 8972-Da peak after CsA administration in GVHD mice. (A) Representative SELDI spectra of control plasma (day 0; before transplantation) and GVHD plasma (days 7, 14, 21, and 28 after transplantation) from the same mouse. The m/z range of 6000 to 10 000 m/z is shown. CsA was administered from day 8 through day 13 after transplantation, indicated by closed triangle. The box highlights a plasma peak with an average mass of 8972 Da that decreased after administration of CsA. The peak intensity of 8972 Da is 5.8, 69.4, 13.8, 15.7, and 14.3 on days 0, 7, 14, 21, and 28, respectively. (B) Mean normalized intensity for the 8972-Da peak in the samples on days 0 (before transplantation), 7, 14, 21, and 28 (n = 6, respectively). The average intensity (± SE) is depicted (n = 6). The 8972-Da peak seen at day 7 significantly decreased at days 14, 21, and 28 (paired t test). The peak at day 7 is significantly higher than that of day 0 (before transplantation) (paired t test). Asterisk indicates P value.
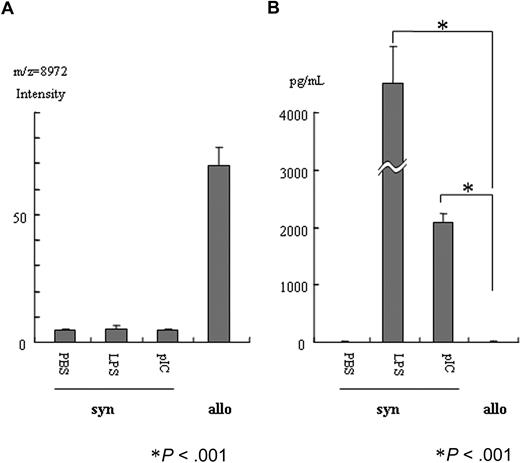
LPS or poly(I:C) treatment did not increase the 8972-Da peak
Figure 3A shows the effect of LPS or poly(I:C) on the 8972-Da peak in syngeneic BMT. Neither LPS nor poly(I:C) increased the 8972-Da peak, although allogeneic marrow transplantation apparently increased it. LPS or poly(I:C) increased serum IL-6 concentration dramatically, although allogeneic marrow transplantation did not increase serum IL-6 at day 7 after transplantation (Figure 3B).
LPS or poly(I:C) treatment did not increase the 8972-Dapeak. (A) At day 7 after transplantation with syngeneic BM cells (syn), mice were treated with LPS, poly(I:C), or PBS. The average value ± SE is depicted (n = 6). No effect of LPS or poly(I:C) on the 8972-Da peak was detectable, although allogeneic marrow (allo) induced an apparent increase of the 8972-Da peak. The average intensity of the peak ± SE was 4.7 ± 0.69, 5.4 ± 1.09, 4.55 ± 0.79, and 69.3 ± 7.02 on PBS, LPS, and poly(I:C) treatment of syngeneic transplantation and untreated allogeneic transplantation, respectively (n = 6). Asterisk indicates P value. (B) LPS or poly(I:C) increased serum IL-6 concentration dramatically. In a clear contrast, concentration of IL-6 was very low in allogeneic samples similar to PBS treatment (unpaired t test). The average concentration (pg/mL) of IL-6 ± SE was 1.38 ± 0.85, 32 215.5 ± 1759.3, 2087 ± 151.3, and 1.25 ± 0.13 on PBS, LPS, and poly(I:C) treatment of syngeneic transplantation and untreated allogeneic transplantation, respectively (n = 6). The difference of PBS treatment and allogeneic transplantation is not statistically significant. Asterisk indicates P value.
LPS or poly(I:C) treatment did not increase the 8972-Dapeak. (A) At day 7 after transplantation with syngeneic BM cells (syn), mice were treated with LPS, poly(I:C), or PBS. The average value ± SE is depicted (n = 6). No effect of LPS or poly(I:C) on the 8972-Da peak was detectable, although allogeneic marrow (allo) induced an apparent increase of the 8972-Da peak. The average intensity of the peak ± SE was 4.7 ± 0.69, 5.4 ± 1.09, 4.55 ± 0.79, and 69.3 ± 7.02 on PBS, LPS, and poly(I:C) treatment of syngeneic transplantation and untreated allogeneic transplantation, respectively (n = 6). Asterisk indicates P value. (B) LPS or poly(I:C) increased serum IL-6 concentration dramatically. In a clear contrast, concentration of IL-6 was very low in allogeneic samples similar to PBS treatment (unpaired t test). The average concentration (pg/mL) of IL-6 ± SE was 1.38 ± 0.85, 32 215.5 ± 1759.3, 2087 ± 151.3, and 1.25 ± 0.13 on PBS, LPS, and poly(I:C) treatment of syngeneic transplantation and untreated allogeneic transplantation, respectively (n = 6). The difference of PBS treatment and allogeneic transplantation is not statistically significant. Asterisk indicates P value.
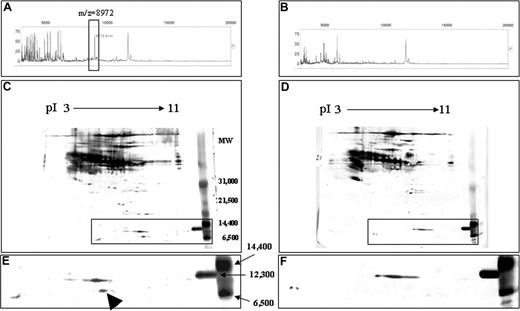
Identification of the 8972-Da protein
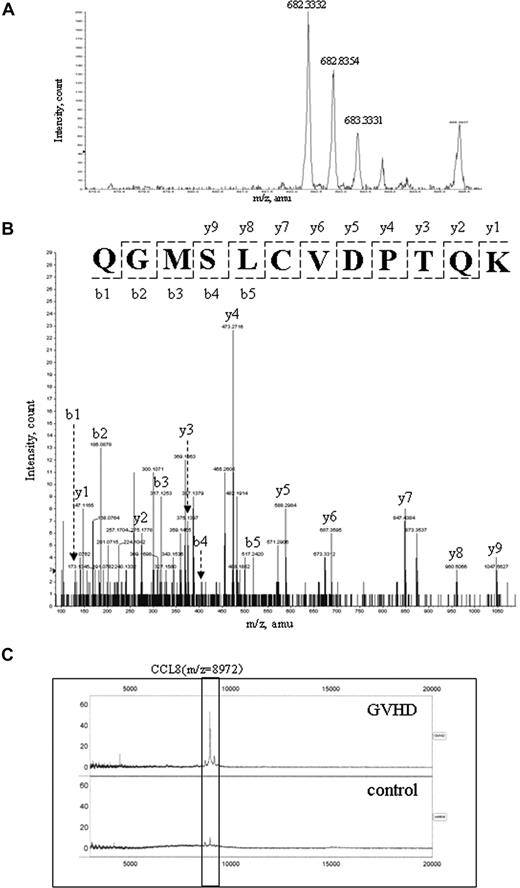
A fraction with an elution time of 31.0 to 31.5 minutes (approximately 38% acetonitrile) contained abundant 8972-Da protein in GVHD samples, but little in controls (Figure 4A,B). The fractions of GVHD and control samples were collected, concentrated, and subsequently loaded onto 2DE. By comparing the 2 gel images of GVHD and control samples, we identified a highly expressed spot with apparent molecular mass of between 6500 Da and 12 300 Da in the gel of GVHD samples (Figure 4C and arrow in 4E), suggesting a relationship between the 8972-Da marker and the 2DE candidate spot. After in-gel digestion, this spot was identified by nanoLC-MS/MS as a CCL8 precursor with MASCOT score 236 (data not shown). One of the MS/MS spectra leading to the identification of CCL8 is presented in Figure 5A,B. Eleven peptides were matched to the theoretic masses with 52% coverage of the amino acid sequence (data not shown). The predicted mass of CCL8 precursor is 11 017 Da and its predicted pI is 8.64. The CCL8 precursor contains a signal peptide of 19 amino acids followed by the mature CCL8 sequence of 78 amino acid residues. The predicted mass of mature CCL8 is 8972.65 with a predicted pI of 8.45, which is consistent with the data acquired by SELDI-TOF MS and 2DE. The identity of the 8972-Da marker as CCL8 was further verified by SELDI immunoassay using a specific rabbit antimouse CCL8 Ab on a PS20 ProteinChip. The assay showed that while CCL8 was captured and detected from GVHD plasma samples, very little was obtained from control samples (Figure 5C).
Purification of 8972-Da protein from murine plasma. Results from GVHD (A,C,E) and control (B,D,F) plasma are shown. (A,B) SELDI-TOF analyses of HPLC fraction 31.0 to 31.5 minutes derived from GVHD (A) and control (B) samples are shown. The box identifies a peak of 8972 Da that is overexpressed in GVHD (A), but not detectable in control plasma (B). (C,D) 2DE gel images of GVHD (C) and control (D) samples are shown. Plasma proteins (70 μg) of HPLC-purified fractions (pool of 31.0- to 31.5-minute fraction) from GVHD and control samples, respectively, were separated on pH 3-11 IPG in the first dimension, followed by SDS-PAGE on 8% to 20% gradient gel in the second dimension. The separated proteins were stained with silver nitrate. Insert is magnified in panels E,F. (E,F) Detailed views with apparent molecular mass of between 6500 and 14 400 Da in the 2DE gel images of GVHD (C) and control (D) samples are shown. Arrowhead indicates the candidate spot for the 8972-Da peak in GVHD (E) sample, which is not apparent in the control (F) sample.
Purification of 8972-Da protein from murine plasma. Results from GVHD (A,C,E) and control (B,D,F) plasma are shown. (A,B) SELDI-TOF analyses of HPLC fraction 31.0 to 31.5 minutes derived from GVHD (A) and control (B) samples are shown. The box identifies a peak of 8972 Da that is overexpressed in GVHD (A), but not detectable in control plasma (B). (C,D) 2DE gel images of GVHD (C) and control (D) samples are shown. Plasma proteins (70 μg) of HPLC-purified fractions (pool of 31.0- to 31.5-minute fraction) from GVHD and control samples, respectively, were separated on pH 3-11 IPG in the first dimension, followed by SDS-PAGE on 8% to 20% gradient gel in the second dimension. The separated proteins were stained with silver nitrate. Insert is magnified in panels E,F. (E,F) Detailed views with apparent molecular mass of between 6500 and 14 400 Da in the 2DE gel images of GVHD (C) and control (D) samples are shown. Arrowhead indicates the candidate spot for the 8972-Da peak in GVHD (E) sample, which is not apparent in the control (F) sample.
Protein identification using mass spectrometry. (A) A representative MS spectrum (682.3332 m/z) of the peptide from the candidate spot in Figure 4C is shown, which was identified by nanoLC-MS/MS. (B) The deduced sequence is confirmed by MS/MS spectra of b and y ion series as CCL8 peptide (QGMSLCVDPTQK) corresponding to 682.3332 parent ion in panel A. Cysteine is carbamidomethylated in this peptide. (C) Verification of CCL8 as the 8972-Da peak by immunoSELDI is shown. The box highlights the elevation of CCL8 in the GVHD sample but not in the control sample.
Protein identification using mass spectrometry. (A) A representative MS spectrum (682.3332 m/z) of the peptide from the candidate spot in Figure 4C is shown, which was identified by nanoLC-MS/MS. (B) The deduced sequence is confirmed by MS/MS spectra of b and y ion series as CCL8 peptide (QGMSLCVDPTQK) corresponding to 682.3332 parent ion in panel A. Cysteine is carbamidomethylated in this peptide. (C) Verification of CCL8 as the 8972-Da peak by immunoSELDI is shown. The box highlights the elevation of CCL8 in the GVHD sample but not in the control sample.
Detection of CCL8 in human GVHD sera
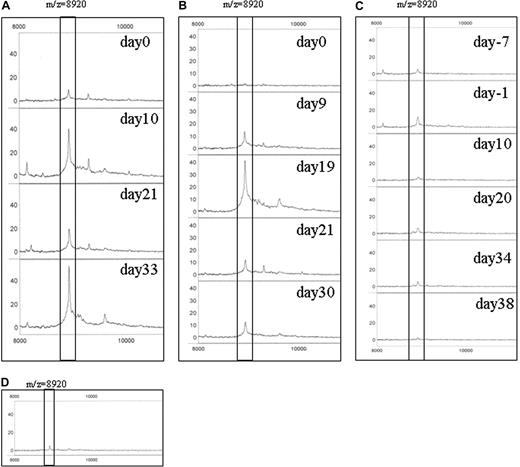
To examine the clinical significance of CCL8 in GVHD sera, we first studied the relative intensity of CCL8 in patients treated with HSCT with or without GVHD. Changes in serum CCL8 peak intensities during the course of HSCT are depicted in Figure 6. Patient A developed clinical GVHD on day 13 after HSCT and started mPSL the same day (Figure 6A). The peak profile of CCL8, which was very weak on day 0 and obviously increased on day 10, had declined by day 21 after mPSL treatment. The peak was again up-regulated by day 33. In patient B, the CCL8 peak was undetectable on day 0, increased on days 9 and 19, and decreased on days 21 and 30 (Figure 6B). Clinical GVHD was recognized on day 19 and mPSL was started. In marked contrast, patient C remained GVHD free throughout her course (Figure 6C). The CCL8 peak was very low in all samples from this patient. In Figure 6D, an example of a healthy control is shown. The CCL8 peak is barely detectable. These data suggest a strong relationship between CCL8 peak and GVHD status in humans.
CCL8 expression in human serum after HSCT. Serum was obtained at several time points after HSCT. The results of immunoSELDI using antibody specific for human CCL8 are shown. Box highlights peak of human CCL8. The predicted molecular mass of mature human CCL8 is 8920 Da. (A) Patient A developed clinical GVHD on day 13 after HSCT and methyl prednisolone (mPSL) was administered the same day. The peak profile of CCL8 was very weak on day 0 with an obvious increase on day 10. CCL8 then declined on day 21 after mPSL treatment, but was again up-regulated on day 33. The peak intensity of 8920 Da was 9.6, 40.6, 19.5, and 52.5 on days 0, 10, 21, and 33, respectively. (B) In patient B, no CCL8 peak was detected on day 0, but was increased on days 9 and 19, and decreased on days 21 and 30. Clinical GVHD was overt on day 19 and mPSL treatment was started. The peak intensity of 8920 Da is 2.1, 14.3, 41.4, 12.3, and 12.7 on days 0, 9, 19, 21, and 30, respectively. (C) No GVHD developed in patient C throughout the course. CCL8 peak was very low in all samples examined. The peak intensity of 8920 Da is 5.8, 12.8, 4.1, 6.5, 6.2, and 3.0 on days −7, −1, 10, 20, 34, and 38, respectively. (D) An example of CCL8 expression in a healthy control is shown. CCL8 peak is very low. The peak intensity of 8920 Da is 5.3.
CCL8 expression in human serum after HSCT. Serum was obtained at several time points after HSCT. The results of immunoSELDI using antibody specific for human CCL8 are shown. Box highlights peak of human CCL8. The predicted molecular mass of mature human CCL8 is 8920 Da. (A) Patient A developed clinical GVHD on day 13 after HSCT and methyl prednisolone (mPSL) was administered the same day. The peak profile of CCL8 was very weak on day 0 with an obvious increase on day 10. CCL8 then declined on day 21 after mPSL treatment, but was again up-regulated on day 33. The peak intensity of 8920 Da was 9.6, 40.6, 19.5, and 52.5 on days 0, 10, 21, and 33, respectively. (B) In patient B, no CCL8 peak was detected on day 0, but was increased on days 9 and 19, and decreased on days 21 and 30. Clinical GVHD was overt on day 19 and mPSL treatment was started. The peak intensity of 8920 Da is 2.1, 14.3, 41.4, 12.3, and 12.7 on days 0, 9, 19, 21, and 30, respectively. (C) No GVHD developed in patient C throughout the course. CCL8 peak was very low in all samples examined. The peak intensity of 8920 Da is 5.8, 12.8, 4.1, 6.5, 6.2, and 3.0 on days −7, −1, 10, 20, 34, and 38, respectively. (D) An example of CCL8 expression in a healthy control is shown. CCL8 peak is very low. The peak intensity of 8920 Da is 5.3.
Elevation of CCL8 in GVHD sera
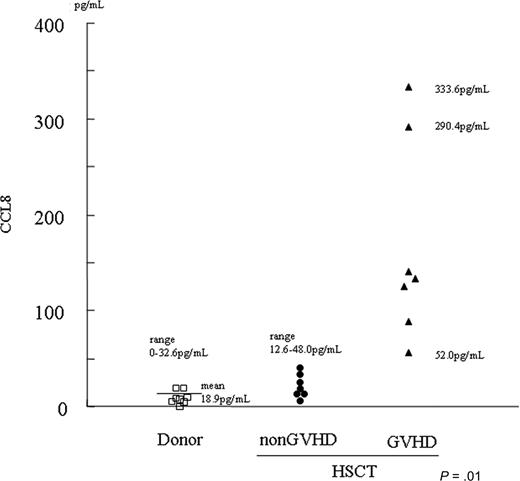
Since the SELDI technique is semiquantitative, we next quantitated CCL8 concentration in patient's sera using ELISA. Patient profiles are summarized in Table 3. As shown in Figure 7, ELISA studies suggested that CCL8 concentration correlates with GVHD in HSCT patients. All non-GVHD plasma contained less than 48 pg/mL (mean ± SE: 22.5 ± 5.5 pg/mL, range: 12.6-48.0 pg/mL, n = 7). In contrast, CCL8 is highly up-regulated in GVHD plasma ranging from 52.0 to 333.6 pg/mL (mean ± SE: 165.0 ± 39.8 pg/mL, n = 7). Of note were 2 patients with severe, eventually fatal, GVHD who had dramatically high levels of CCL8 (333.6 and 290.4 pg/mL). In healthy controls, the mean concentration of CCL8 was 18.9 pg/mL (range: 0.00 to 32.6 pg/mL, n = 8).
Patient characteristics
| . | No. . |
|---|---|
| N | 14 |
| Sex | |
| Male | 7 |
| Female | 7 |
| Diagnosis | |
| ALL | 7 |
| CML | 1 |
| Aplastic anemia | 3 |
| Fanconi anemia | 1 |
| CGD | 1 |
| HLH | 1 |
| Donor | |
| Related | 8 |
| Unrelated | 6 |
| Conditioning regimen | |
| TBI + CY | 5 |
| TBI + Flu | 2 |
| TBI + VP + CY | 1 |
| Flu + CY | 3 |
| VP + BU + CY | 1 |
| CA + BU + VP + L-PAM | 1 |
| TBI + L-PAM | 1 |
| GVHD prophylaxis | |
| CsA + MTX | 5 |
| FK + MTX | 3 |
| CsA + MMF | 2 |
| CsA | 1 |
| MTX | 2 |
| CsA + MTX + mPSL | 1 |
| . | No. . |
|---|---|
| N | 14 |
| Sex | |
| Male | 7 |
| Female | 7 |
| Diagnosis | |
| ALL | 7 |
| CML | 1 |
| Aplastic anemia | 3 |
| Fanconi anemia | 1 |
| CGD | 1 |
| HLH | 1 |
| Donor | |
| Related | 8 |
| Unrelated | 6 |
| Conditioning regimen | |
| TBI + CY | 5 |
| TBI + Flu | 2 |
| TBI + VP + CY | 1 |
| Flu + CY | 3 |
| VP + BU + CY | 1 |
| CA + BU + VP + L-PAM | 1 |
| TBI + L-PAM | 1 |
| GVHD prophylaxis | |
| CsA + MTX | 5 |
| FK + MTX | 3 |
| CsA + MMF | 2 |
| CsA | 1 |
| MTX | 2 |
| CsA + MTX + mPSL | 1 |
ALL indicates acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CGD, chronic granulomatous disease; HLH, hemophagocytic lymphohistiocytosis; TBI, total body irradiation; CY, cyclophosphamide; Flu, fludarabine; VP, etoposide; BU, busulfan; L-PAM, melphalan; CsA, cyclosporin A; MTX, methotrexate; FK, tacrolimus; MMF, mycofenolate mofetil; and mPSL, methyl prednisolone.
Concentration of CCL8 in human sera. Concentration of CCL8 in human sera was determined by ELISA for human CCL8. All sera from those who did not develop GVHD after HSCT (non-GVHD/HSCT) contained less than 48 pg/mL (mean ± SE: 22.5 ± 5.5 pg/mL, range: 12.6-48.0 pg/mL, n = 7). CCL8 was highly up-regulated in GVHD plasma (GVHD/HSCT) ranging from 52.0 to 333.6 pg/mL (mean ± SE: 165.0 ± 39.8 pg/mL, n = 7). Two patients with severe, eventually fatal, GVHD showed extremely high levels of CCL8 (ie, 333.6 and 290.4 pg/mL). In healthy controls (donors), mean concentration of CCL8 was 18.9 pg/mL (range: 0.00 to 32.6 pg/mL, n = 8). The difference in serum concentration of CCL8 was statistically significant between non-GVHD and GVHD plasma obtained from patients undergoing HSCT.
Concentration of CCL8 in human sera. Concentration of CCL8 in human sera was determined by ELISA for human CCL8. All sera from those who did not develop GVHD after HSCT (non-GVHD/HSCT) contained less than 48 pg/mL (mean ± SE: 22.5 ± 5.5 pg/mL, range: 12.6-48.0 pg/mL, n = 7). CCL8 was highly up-regulated in GVHD plasma (GVHD/HSCT) ranging from 52.0 to 333.6 pg/mL (mean ± SE: 165.0 ± 39.8 pg/mL, n = 7). Two patients with severe, eventually fatal, GVHD showed extremely high levels of CCL8 (ie, 333.6 and 290.4 pg/mL). In healthy controls (donors), mean concentration of CCL8 was 18.9 pg/mL (range: 0.00 to 32.6 pg/mL, n = 8). The difference in serum concentration of CCL8 was statistically significant between non-GVHD and GVHD plasma obtained from patients undergoing HSCT.
Discussion
HSCT can be curative for various hematologic, oncologic, metabolic, and immune disorders. However, GVHD remains a major life-threatening complication after transplantation. Early and sensitive detection of GVHD and the ability to monitor treatment efficacy should improve the outcome for patients after HSCT.
Recently, proteomics has emerged as a key technique to identify biomarkers and disease targets. To discover novel biomarkers for GVHD, we used SELDI-TOF MS, which is one of several high-throughput technologies able to generate peptide and protein profiles of complex biologic samples with high sensitivity, reproducibility, and simplicity. Using the SELDI technique, we first compared plasma protein profiles between control and GVHD mice. Since human samples have intrinsic person-to-person variability that may obscure the discovery of new biomarkers, we considered that the relative simplicity of an animal model of GVHD would give better analytic power including estimates of specificity and sensitivity. We selected an 8972-Da protein as a potential plasma biomarker for GVHD in mice and also determined its expression in syngeneic transplantation, and after CsA, LPS, or poly(I:C) treatment. The 8972-Da expression was not increased in syngeneic combination mice, suggesting that it is not induced by pretreatment regimens given before bone marrow transplantation including radiation. Decrease of the 8972-Da peak expression following CsA administration in GVHD mice was strongly correlated with GVHD activity. Furthermore, neither LPS nor poly(I:C) induced an 8972-Da peak. These results suggest the probable existence of a protein or proteins linked to the onset and severity of GVHD at least in mouse plasma. We identified this peak as CCL8, one of the CC chemokines.
As observed in the mice, we found elevated serum levels of CCL8 in humans with GVHD but not in non-GVHD patients who had undergone HSCT. These results suggest that CCL8 is a potential biomarker for human GVHD. Human CCL8 has 2 forms: one has 76 amino acids (1-76) with biologic activity, and the other has 71 amino acids (6-76) and no activity.16 We detected a single peak with 8920 Da (average mass). Since the biologic role of human CCL8 is not well characterized, it should be determined whether this peak is derived from 1-76 or 6-76 CCL8. Before concluding that CCL8 is a valid biomarker for GVHD, we must determine whether it differentiates GVHD from other complications with overlapping clinical features, such as veno-occulusive disease (VOD), or viral reactivation. In preliminary studies, we found that CCL8 was not elevated in 2 cases of VOD (data not shown). A previous study demonstrated that CCL8 levels were increased in patients with Gram-positive sepsis.17 Additional studies with larger populations of human patients after HSCT are currently under way and are essential to verify the role of CCL8 as a biomarker for human GVHD.
Chemokines are predominantly small molecules (8 to 12 kDa) that bind to G protein–coupled receptors (chemokine receptors) and function primarily in leukocyte migration.18 The chemokine superfamily currently comprises more than 50 members subclassified into 4 families based on the arrangement of their cysteine residues: CXC, C, CX3C, and CC.19 CCL8 is also known as monocyte chemoattractant protein-2 (MCP-2). CCL2/MCP-1, CCL7/MCP-3, CCL8/MCP-2, and CCL13/MCP4 constitute a small subfamily within the CC chemokine group, which acts as a major chemoattractant for monocytes, activated T cells, and dendritic cells.20,21 Chemokine and chemokine receptor interactions play a crucial role in donor T-cell migration in GVHD.22-27 However none of the above studies addressed the role of CCL8. The only report connecting CCL8 to GVHD was by Sugerman et al who showed up-regulated gene expression of CCL8 in mouse cutaneous GVHD.28
GVHD is initiated by allogeneic activation of donor T cells by host-derived antigen presenting cells (APCs).9 Dendritic cells (DCs) are professional APCs that activate naive T cells most efficiently, and CCL8 is one of the chemokines secreted by mature DCs. These findings are consistent with our demonstration that CCL8 expression is up-regulated during GVHD. Although the complex interactions of chemokines involved in the induction of GVHD remain poorly understood, CCL8 is thought to be pivotal. CCL8 as a biomarker may facilitate early diagnosis of GVHD and assessment of treatment response, since it seems to be engaged in the development and progression of GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Peter M. Olley for helpful discussion and English revision of our paper.
This study was supported by grants from the Ministry of Health, Labor and Welfare of Japan and from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authorship
Contribution: T.H., Y.N., H.S., and Y.M. performed research; T.S. performed statistical analysis; N.S., N.H., M.Y., K.I., and H.T.contributed vital new reagents or analytic tools; Y.K. designed research; T.H. and Y.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yasuo Kokai, Department of Biomedical Engineering, Sapporo Medical University School of Medicine, S1W17, Chuo-ku, Sapporo, 060-8556, Japan; e-mail: kokai@sapmed.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal