To the editor:
The recent publication by Schade et al1 demonstrated that the Src/Abl kinase inhibitor dasatinib (Sprycel), used for the treatment of chronic myeloid leukemia,2 inhibits the function of T cells in vitro and in vivo. Dasatinib inhibition of the Src-family kinase LCK, which is critical for T-cell receptor (TCR) signaling, was proposed as the mechanism for inhibited T-cell function. To extend our understanding of the potential impact of dasatinib on the immune system we have evaluated the effects of dasatinib on natural killer (NK) cells.
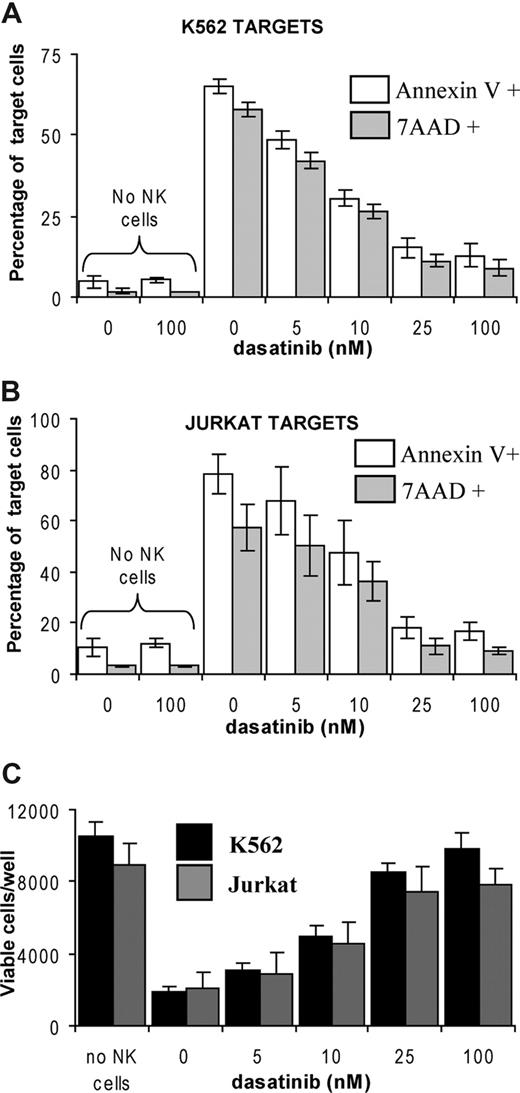
NK cells were isolated from normal blood through negative immunomagnetic isolation (Miltenyi Biotec, Auburn, CA) after density centrifugation to isolate peripheral blood mononuclear cells (PBMCs). Normal blood was collected with informed consent according to the Declaration of Helsinki and experiments were approved by the Human Ethics Committee, Royal Adelaide Hospital (Adelaide, Australia). NK cell activity was assessed by measuring cytotoxicity against the K562 and Jurkat cell lines. Cytotoxicity was assessed by flow cytometry using a modification of the method described in Hoppner et al.3 NK cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) as described previously4 and cultured at a 10:1 ratio with target cells in the presence of 0 to 100 nM dasatinib. After 3 hours the proportion of apoptotic and dead cells in the CFSE–negative target population was determined by annexin V (BD Biosciences, San Jose, CA) and 7-amino-actinomycin D (7AAD; Invitrogen) staining and cell counts were also performed. As shown in Figure 1, dasatinib reduced NK cell cytotoxicity in a dose dependent manner with maximal effect achieved at approximately 25 nM. While potently inhibited, NK function was not completely blocked by dasatinib with approximately 10% cytotoxicity against target cells observed at 100 nM. The effect of dasatinib on NK function was largely reversible as washing NK cells to remove dasatinib after a 3-hour incubation with 25 nM dasatinib restored approximately 90% cytotoxicity against K562 cells compared with untreated control cells (data not shown). The viability of both cell lines was not affected by dasatinib alone during the short drug exposure in these experiments (Figure 1).
NK cell cytotoxicity is reduced in the presence of dasatinib. CFSE-labeled NK cells were cultured at a 10:1 ratio with cell lines K562 (A) or Jurkat cell targets (B). After 3 hours incubation samples were stained with annexin V or 7AAD and analyzed on a flow cytometer; plots are representative of the percentage of each cell line staining positive for annexin V or 7AAD. A fixed number of beads were added to each sample to allow the total number of CFSE-negative target and CFSE-positive NK cells to be determined after flow analysis. From this the total number of viable K562s or Jurkat cells was determined (C) where nonviable cells were defined by annexin V positivity. Means and standard deviations are shown from at least 3 separate experiments using different healthy donors.
NK cell cytotoxicity is reduced in the presence of dasatinib. CFSE-labeled NK cells were cultured at a 10:1 ratio with cell lines K562 (A) or Jurkat cell targets (B). After 3 hours incubation samples were stained with annexin V or 7AAD and analyzed on a flow cytometer; plots are representative of the percentage of each cell line staining positive for annexin V or 7AAD. A fixed number of beads were added to each sample to allow the total number of CFSE-negative target and CFSE-positive NK cells to be determined after flow analysis. From this the total number of viable K562s or Jurkat cells was determined (C) where nonviable cells were defined by annexin V positivity. Means and standard deviations are shown from at least 3 separate experiments using different healthy donors.
Src-family kinases play an important role coupling receptor activation to intracellular signaling in NK cells. Unlike T-cells where LCK plays a critical role in TCR signaling, there is greater redundancy in NK cell signaling pathways and a number of Src-family kinases including LCK, Fyn, and Src are involved.5-7 Thus, the broad Src-kinase inhibitory activity of dasatinib is likely to be necessary for inhibition of NK cytotoxicity. Additionally the Src-kinase inhibitor (PP2) has previously been shown to significantly reduce NK cell cytotoxicity.8 As NK cells provide a first line of defense against viruses and provide surveillance against tumors, their inhibition is of clinical relevance. As shown by Schade, inhibition of T-cells by dasatinib is achievable in murine models, but the doses required were higher than those required to inhibit CML models.9 Thus, if the in vivo effects of dasatinib against NK cells are similar to those against T-cells, once-daily dosing with dasatinib may result in only minor suppression of NK cell function and greater suppression might occur when dasatinib is taken at higher doses or with greater frequency. Further in vivo studies expanding on our findings will assist in discerning any effects of dasatinib on NK cell function in patients.
Authorship
We thank Bristol Myers Squibb for providing the dasatinib for use in these experiments.
Funding was provided through an Adelaide University, Molecular Life Sciences PhD divisional scholarship.
Conflict-of-interest disclosure: T.P.H. is on the advisory board of Bristol Myers Squibb and also receives research funding. The other authors declare no competing financial interests.
Correspondence: Stephen J. Blake, Institute of Medical and Veterinary Science, Frome Road, Adelaide, Australia; e-mail: stephen.blake@imvs.sa.gov.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal