Based on observations that IL-21 stimulates apoptosis in CLL cells (see figure) and additively enhances the cytotoxicity of fludarabine or rituximab, Gowda and colleagues propose the introduction of IL-21 in combination therapy with fludarabine and rituximab.
Chronic lymphocytic leukemia (CLL) is still considered an incurable disease, despite recent progress due to the development of agents such as fludarabine and antibodies (rituximab, alemtuzumab); thus, additional therapeutic approaches are highly desired.
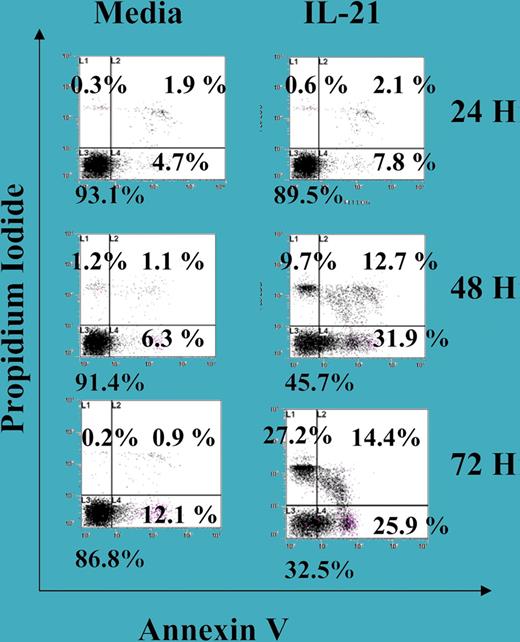
IL-21–induced cell death in B-CLL cells. See the complete figure in the article beginning on page 4723.
IL-21–induced cell death in B-CLL cells. See the complete figure in the article beginning on page 4723.
IL-21 triggers the antibody-dependent cellular cytotoxicity (ADCC) exerted by natural killer (NK) cells on rituximab-coated syngeneic or allogeneic CLL cells in all patients, including those resistant to apoptosis induction by IL-21. IL-21 appears, therefore, more interesting than other NK-stimulatory cytokines, such as IL-2 and IL-12, that present the disadvantage of simultaneously favoring the proliferation of CLL cells and/or preventing their apoptosis. It is of note that IL-21 does not potentiate fludarabine-mediated cytotoxicity in T cells, thereby avoiding the risk of cellular immune suppression.
IL-21 is a member of the 4 helix bundle family that signals through IL-21Rα in association with the common γ-chain.1 Inasmuch as the level of IL-21–driven apoptosis in CLL cells is correlated with membrane expression of IL-21Rα, cytotoxicity could be enhanced by agents known to increase IL-21Rα expression, such as CD40 ligand or CpG oligonucleotides.
IL-21–stimulated apoptosis of CLL cells requires the up-regulation of Bim, a BH3-only member of the Bcl-2 family. This role of Bim is of utmost interest, in light of the recently described hierarchical regulation of mitochondrion-dependent apoptosis by Bcl-2 subfamilies.2 In this model, a subset of BH3-only inactivator proteins binds members of the antideath Bcl-2 family and induces them to release prodeath binding partners. The latter are members of the other subset of BH3-only activator proteins, including Puma and tBid (truncated Bid) in addition to Bim. Similarly to tBid, Bim and Puma release cytochrome c from mitochondria, either directly or through the displacement of Bak from the outer mitochondrial membrane channel VDAC2. The mechanism of the up-regulation of Bim and the identification of its partners would therefore be worthy of further investigation. In order to increase the level of apoptosis in B-CLL cells, it would also be interesting to combine IL-21 with treatment aimed at stimulating the expression of the other BH3-only activator proteins, tBid and Puma.
IL-21 was previously reported to be a growth and survival factor for human multiple myeloma (MM) cells.3 A clue for explaining this discrepancy with CLL could reside in the balance between the tyrosine phosphorylation of Stat1 (enhancing apoptosis) and of Stat3 (antagonizing Stat1 and apoptosis); whereas IL-21 elicits Stat3 tyrosine phosphor-ylation in CLL and MM cells, no phosphorylation of Stat1 is observed in MM, at variance with responder CLL cells.
A recent report of a phase 1 trial of IL-21 in patients with metastatic melanoma has concluded that this cytokine is biologically active and generally well tolerated, with a maximum tolerated dose (MTD) of 30 μg/kg.4 Therefore, clinical trials combining IL-21 with agents commonly utilized as initial treatment in CLL could be envisaged in the near future.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal