Abstract
Gene expression profiling and immunohistochemical studies have demonstrated that nonmalignant tumor infiltrating inflammatory cells contribute to clinical outcome in patients with follicular lymphoma (FL). Particularly, tumor-associated macrophage (TAM) content correlates with longer survival rates after immunochemotherapy. Here we investigated the prognostic importance of tumor-associated mast cells (MCs) and their relation to TAMs in patients with FL treated with a combination of rituximab (R) and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy. Of the 98 patients, 70 received R-CHOP at diagnosis and 28 at relapse. According to Kaplan-Meier estimates, the patients with high MC content had a worse 4-year progression-free survival (PFS) than the ones with low MC content after R-CHOP therapy (34% vs 74%, P = .002). The adverse prognostic value of MCs was seen both for the patients treated at diagnosis and at relapse, whereas no such impact on PFS was observed for the control patients treated with chemotherapy only (P = .4). When the TAM-related PFS was analyzed separately in patients with high and low MC contents, the positive prognostic effect of TAM was seen only in patients with few MCs. Taken together, the data demonstrate that a high MC score is associated with unfavorable prognosis and it eliminates the positive prognostic value of TAMs in patients with FL treated with immunochemotherapy.
Introduction
Follicular lymphoma (FL) is the second most common subtype of all non-Hodgkin lymphomas (NHLs). The clinical course of the disease is mainly indolent but relapses are frequent, and eventually resistance to chemotherapy or transformation to aggressive lymphoma occurs, and the patients die as a result of their disease.1 A number of attempts to improve the survival rates have proven unsuccessful. Recently, however, a significant improvement of the outcome of patients has been obtained by combining a monoclonal anti-CD20 antibody, rituximab (R), with induction chemotherapy, or by prolonging the remission with R maintenance therapy.2-7 Despite the advances, response to treatment varies considerably among individual patients and outcome is often unpredictable
To date, the Follicular Lymphoma International Prognostic Index (FLIPI) is the only valuable prognostic tool specifically designed for patients with FL.8 Although FLIPI was developed before R was established in the treatment of FL, it can also be used as a predictor of the outcome after R-CHOP regimen.9 However, FLIPI alone cannot accurately predict the outcome of individual patients and only indirectly reflects FL biology. Therefore, additional prognostic tools would be appreciated.
In recent years, microarray-based techniques have provided valuable prognostic information on distinct lymphomas. In FL, the genes associated with infiltrating inflammatory cells seem to be more important than the tumor cells themselves for predicting the outcome.10,11 At the cellular level, tumor-infiltrating cytotoxic and regulatory T cells, and macrophages, have prognostic impact in FL12-16 after chemotherapy. However, their prognostic importance needs to be re-evaluated in the post-R era of lymphoma therapies. We have previously shown that addition of R to chemotherapy reverses the negative prognostic impact of high macrophage content to favorable.17 Similarly, Canioni et al18 have recently reported that R is able to circumvent the unfavorable outcome associated with high macrophage content in cyclophosphamide, doxorubicin, etoposide, prednisone, and interferon (CHVP-I)–treated patients. The present study provides evidence that MC infiltration is an unfavorable prognostic factor in FL after R-CHOP regimen.
Methods
Patients
The study population consisted of 98 patients with FL treated with a combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in front-line or first relapse (Table 1). All patients received R for the first time. The relapsed patients had received various treatments front-line, including chlorambucil, combination chemotherapy, and local irradiation. All tissue samples were taken before R-CHOP. Lymphoma classifications including histopathology and immunophenotyping were performed at the Department of Pathology at Helsinki University Central Hospital Laboratory Diagnostics (HUSLAB) according to the World Health Organization (WHO) classification. Treatment records of all patients were reviewed to confirm the appropriate treatment protocols and to document clinical characteristics, prognostic factors, and long-term follow-up. The protocol and sampling were approved by the institutional review board and the Finnish National Authority for Medicolegal Affairs.
Characteristics of R-CHOP–treated and control patients (pre-R group) with FL according to MC content
| Characteristic . | R-CHOP group . | Control group (pre-R group) . | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients, n = 98 . | Low MC content (≤ median), n = 51 . | High MC content (> median), n = 47 . | P . | All patients, n = 28 . | Low MC content, (≤ median), n = 15 . | High MC content, (> median), n = 13 . | P . | |
| Median MC content (range) | 1.5 (0-19) | 0.7 (0-1.5) | 3.8 (1.7-19) | NA | 1.5 (0-19) | 0.67 (0.4-10) | 3.8 (1.7-19) | NA |
| Mean MC content (SD) | 2.7 (3.4) | 0.7 (0.53) | 4.9 (3.8) | 2.9 (4.0) | 0.7 (0.50) | 5.4 (4.8) | ||
| Sex, n (%) | .064 | 1.000 | ||||||
| Female | 58 (59) | 35 (67) | 23 (49) | 18 (64) | 10 (67) | 8 (62) | ||
| Male | 40 (41) | 16 (31) | 24 (51) | 10 (36) | 5 (33) | 5 (38) | ||
| Age, n (%) | 1.000 | 1.000 | ||||||
| Younger than 60 y | 66 (67) | 34 (67) | 32 (68) | 23 (82) | 12 (80) | 11 (85) | ||
| 60 y or older | 32 (33) | 17 (33) | 15 (32) | 5 (18) | 3 (20) | 2 (15) | ||
| State of disease, n (%) | 1.000 | NA | ||||||
| Primary | 70 (71) | 36 (71) | 34 (72) | 28 (100) | 15 (100) | 13 (100) | ||
| Relapse | 28 (29) | 15 (29) | 13 (28) | 0 (0) | 0 (0) | 0 (0) | ||
| Grade, n (%) | .743 | 1.000 | ||||||
| I to II | 88 (90) | 45 (88) | 43 (91) | 26 (93) | 14 (93) | 12 (92) | ||
| III | 10 (10) | 6 (12) | 4 (9) | 2 (7) | 1 (7) | 1 (8) | ||
| R-FLIPI, n (%) | .114 | .639 | ||||||
| 0 to 2 | 62 (63) | 36 (70) | 26 (55.5) | 23 (82) | 13 (87) | 10 (77) | ||
| 3 to 5 | 29 (30) | 11 (22) | 18 (38.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Missing | 7 (7) | 4 (8) | 3 (6) | 5 (18) | 2 (13) | 3 (23) | ||
| Tryptase, n (%) | <.001 | .008 | ||||||
| Median 6.6 or lower | 38 (52) | 28 (76) | 10 (28) | 9 (43) | 8 (73) | 1 (10) | ||
| Median greater than 6.6 | 35 (48) | 9 (24) | 26 (72) | 12 (57) | 3 (27) | 9 (90) | ||
| Characteristic . | R-CHOP group . | Control group (pre-R group) . | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients, n = 98 . | Low MC content (≤ median), n = 51 . | High MC content (> median), n = 47 . | P . | All patients, n = 28 . | Low MC content, (≤ median), n = 15 . | High MC content, (> median), n = 13 . | P . | |
| Median MC content (range) | 1.5 (0-19) | 0.7 (0-1.5) | 3.8 (1.7-19) | NA | 1.5 (0-19) | 0.67 (0.4-10) | 3.8 (1.7-19) | NA |
| Mean MC content (SD) | 2.7 (3.4) | 0.7 (0.53) | 4.9 (3.8) | 2.9 (4.0) | 0.7 (0.50) | 5.4 (4.8) | ||
| Sex, n (%) | .064 | 1.000 | ||||||
| Female | 58 (59) | 35 (67) | 23 (49) | 18 (64) | 10 (67) | 8 (62) | ||
| Male | 40 (41) | 16 (31) | 24 (51) | 10 (36) | 5 (33) | 5 (38) | ||
| Age, n (%) | 1.000 | 1.000 | ||||||
| Younger than 60 y | 66 (67) | 34 (67) | 32 (68) | 23 (82) | 12 (80) | 11 (85) | ||
| 60 y or older | 32 (33) | 17 (33) | 15 (32) | 5 (18) | 3 (20) | 2 (15) | ||
| State of disease, n (%) | 1.000 | NA | ||||||
| Primary | 70 (71) | 36 (71) | 34 (72) | 28 (100) | 15 (100) | 13 (100) | ||
| Relapse | 28 (29) | 15 (29) | 13 (28) | 0 (0) | 0 (0) | 0 (0) | ||
| Grade, n (%) | .743 | 1.000 | ||||||
| I to II | 88 (90) | 45 (88) | 43 (91) | 26 (93) | 14 (93) | 12 (92) | ||
| III | 10 (10) | 6 (12) | 4 (9) | 2 (7) | 1 (7) | 1 (8) | ||
| R-FLIPI, n (%) | .114 | .639 | ||||||
| 0 to 2 | 62 (63) | 36 (70) | 26 (55.5) | 23 (82) | 13 (87) | 10 (77) | ||
| 3 to 5 | 29 (30) | 11 (22) | 18 (38.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Missing | 7 (7) | 4 (8) | 3 (6) | 5 (18) | 2 (13) | 3 (23) | ||
| Tryptase, n (%) | <.001 | .008 | ||||||
| Median 6.6 or lower | 38 (52) | 28 (76) | 10 (28) | 9 (43) | 8 (73) | 1 (10) | ||
| Median greater than 6.6 | 35 (48) | 9 (24) | 26 (72) | 12 (57) | 3 (27) | 9 (90) | ||
For the pre-R group, initial diagnostic FLIPI scores are shown.
R-FLIPI indicates Follicular Lymphoma Prognostic Index before R-CHOP therapy; and NA, not applicable.
Stainings
Formalin-fixed, paraffin-embedded tissue sections were either individual sections or part of a tissue microarray (TMA).17 MCs were detected with a histochemical Leder stain for naphthol-ASD-chloroacetate esterase.19 The number of MCs was counted from 2 to 3 fields per sample at ×400 magnification with a Leica DM LB bright-field microscope (Leica Microsystems, Wetzlar, Germany). To confirm the correlation between the data from TMA (n = 56) and whole-tissue sections (n = 42), the MC frequencies were first analyzed separately between the groups. Because the observed median of 1.5 (used as a cutoff value) was exactly the same in both groups, the samples were further analyzed as one group. To confirm the data on Leder stainings, 73 whole-tissue sections were stained with antihuman tryptase, as previously described.20 A larger surface area allowed quantification of 5 fields per sample. The detection of CD68+ TAMs and their quantification were also as previously described.17
Statistics
To evaluate the correlation between the data from Leder- and tryptase-positive MCs, a Spearman correlation coefficient (rs) was calculated. The Chi square test was used to assess differences in the frequency of prognostic factors. Survival rates were estimated by the Kaplan-Meier method and the differences were compared by the log rank test. PFS was defined as the interval between the start of treatment and documentation of progressive disease. Overall survival (OS) was measured from the start of therapy until the last follow-up or death from any cause. Prognostic impact of identified factors on PFS and OS was analyzed as continuous variables in Cox regression analyses. Both univariate and multivariate analyses were performed. Probability values below .05 were considered statistically significant. All P values were 2-tailed.
Results
The baseline characteristics of the R-CHOP–treated patients are listed in Table 1. The median age of the cohort was 56 years (range, 27-79 years). The median follow-up from diagnosis was 56 months for all patients, 57 months for front-line–treated patients, and 53 months for patients treated in relapse. The patients were further divided into 2 subgroups based on the number of tumor infiltrating Leder stain–positive MCs. The majority of the MCs were located interfollicularly. The mean and the median MC counts were 2.7 and 1.5 (standard deviation [SD] 3.4; range, 0-19), respectively. When the median MC count was used as a cutoff value between 2 groups (low vs high), no differences in age, sex, state of the disease, and FLIPI scores were observed (Table 1).
To confirm the data on Leder stainings, a subset of 73 samples was stained and evaluated for MC tryptase immunoreactivity. The mean and median tryptase-positive MC counts were 7.4 and 6.6 (SD 4.4; range, 0-28), respectively. A subsequent comparison of MC counts demonstrated a significant correlation between Leder stain and tryptase-positive MCs (rs = 0.658; P < .001).
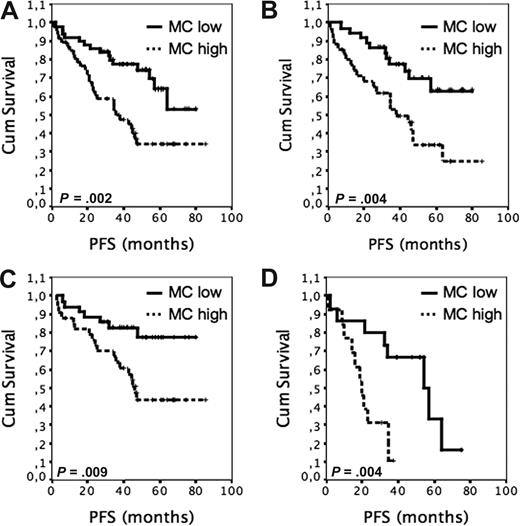
At the median follow-up of 56 months, 85 of 98 patients were alive (87%) and 54 were in remission (55%). PFS after R-CHOP regimen was significantly worse among the patients with high (> median) Leder stain–positive MC scores than it was among those patients with low (≤ median) scores (Figure 1A, 4-year PFS, 34% vs 74%, median PFS 36 months vs median PFS not reached, P = .002). The inverse association of MCs with PFS was confirmed by using tryptase-positive counts in survival analyses. The 4-year PFS for patients with low tryptase-positive MC content (≤ median) was 70% compared with 34% for those with high tryptase-positive cell counts (> median; P = .004; Figure 1B). When the outcome was adjusted for FLIPI, the MC count remained a predictor for PFS in both low to intermediate (FLIPI 0-2), and high (FLIPI 3-5) risk groups (P = .003). The estimated proportion of patients alive at 4 years was 89% for patients with low MC counts, as compared with 91% for those with high MC scores (P = .34).
The outcome of R-CHOP–treated patients with FL according to tumor-infiltrating MC content. (A) PFS of 98 R-CHOP–treated patients according to high (> median 1.5; n = 47) and low (≤ 1.5; n = 51) Leder stain–positive MC content. (B) PFS of 73 R-CHOP–treated patients according to high (> 6.6; n = 35) and low (≤ 6.6; n = 38) tryptase-positive MC content. (C) PFS after front-line R-CHOP according to high (n = 34) and low (n = 36) Leder stain–positive MC content. (D) PFS of patients treated with immunochemotherapy at relapse according to high (n = 13) and low (n = 15) Leder stain–positive MC content.
The outcome of R-CHOP–treated patients with FL according to tumor-infiltrating MC content. (A) PFS of 98 R-CHOP–treated patients according to high (> median 1.5; n = 47) and low (≤ 1.5; n = 51) Leder stain–positive MC content. (B) PFS of 73 R-CHOP–treated patients according to high (> 6.6; n = 35) and low (≤ 6.6; n = 38) tryptase-positive MC content. (C) PFS after front-line R-CHOP according to high (n = 34) and low (n = 36) Leder stain–positive MC content. (D) PFS of patients treated with immunochemotherapy at relapse according to high (n = 13) and low (n = 15) Leder stain–positive MC content.
PFS rates were also estimated separately after front- and second-line therapies. The PFS rates from the date of the first front-line R-CHOP were worse for the patients with many MCs than for the patients with few MCs (Figure 1C; median PFS 46 months vs PFS not reached P = .009). When the outcome of28 patients receiving R-CHOP at their first relapse was analyzed, high MC content was also associated with worse PFS (Figure 1D; median PFS 20 months vs 54 months, P = .004). In contrast, no significant prognostic impact of MC counts analyzed from the same samples was observed on the PFS of the same patient cohort receiving front-line therapy before R was available in clinical routine (pre-R era; 41 months vs 34 months, P = .4). Of note, the PFS curves showed an opposite trend toward improved outcome for the patients with many MCs. The baseline characteristics for the pre-R group are shown in Table 1. When the median MC count was used as a cutoff value between 2 groups, no differences in age, sex, state of the disease, and FLIPI scores were observed (Table 1). In comparison with R-CHOP–treated patients, the pre-R group contained more low- and intermediate-risk patients according to FLIPI at diagnosis (82% vs 64%, P = .002). They also tended to be younger at the time of primary therapy (P = .058). For all other comparisons, no differences were seen.
To further assess the prognostic value of tumor-infiltrating MCs in the post-R era, Cox univariate analyses were performed. Besides previously identified TAM content (P = .022)17 and FLIPI score before R-CHOP therapy (P = .028), MC count was a significant prognostic factor for PFS (P = .007). When the score was included in the multivariate analyses with FLIPI and TAM counts, all factors had independent prognostic value for PFS (Table 2). In the univariate analysis for OS, the prognostic influence of MC score was not significant (P = .065).
Cox proportional hazard regression analysis
| PFS factor . | Relative risk . | 95% confidence interval . | P . |
|---|---|---|---|
| R-FLIPI | 1.310 | 1.006-1.706 | .045 |
| MC score | 1.077 | 1.001-1.159 | .048 |
| TAM score | 0.966 | 0.939-0.995 | .020 |
| PFS factor . | Relative risk . | 95% confidence interval . | P . |
|---|---|---|---|
| R-FLIPI | 1.310 | 1.006-1.706 | .045 |
| MC score | 1.077 | 1.001-1.159 | .048 |
| TAM score | 0.966 | 0.939-0.995 | .020 |
For FLIPI, the higher number is worse. MC and TAM counts were included in the analysis as continuous variables; high MC score is worse than a low MC score, whereas low TAM score is worse than a high TAM score.
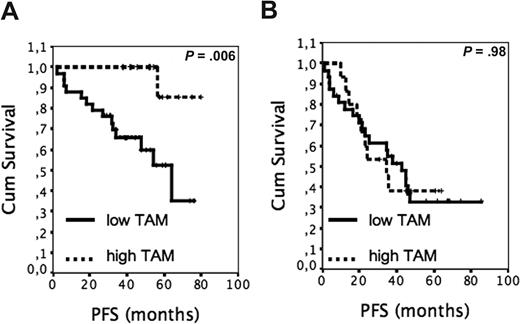
Finally, we investigated whether the negative survival effect of high MC counts interfered with the previously identified predictive value of TAMs.17 The TAM-related PFS was estimated separately for patients with low and high MC content. Among the patients with low MC counts, the PFS was found to be worse in the group with low TAM scores (≤ upper tertile) than in the group with high TAM scores (> upper tertile; Figure 2A, 4-year PFS 60% vs 100%, median PFS 64 months vs PFS not reached, P = .006). In contrast, the effect of TAM content on PFS was not significant for the patients with many MCs (Figure 2B, median PFS 43 months vs 35 months, P = .98).
The outcome of R-CHOP–treated patients with FL according to tumor-infiltrating MCs and TAMs. (A) PFS of patients with low MC scores according to high (n = 17) and low (n = 34) TAM content. (B) PFS of patients with high MC scores according to high (n = 15) and low (n = 32) TAM content.
The outcome of R-CHOP–treated patients with FL according to tumor-infiltrating MCs and TAMs. (A) PFS of patients with low MC scores according to high (n = 17) and low (n = 34) TAM content. (B) PFS of patients with high MC scores according to high (n = 15) and low (n = 32) TAM content.
Discussion
MC infiltration has been detected in different tumors, including lymphomas. In chemotherapy-treated diffuse large B-cell lymphoma (DLBCL), the patients with many tumor-infiltrating MCs have a significantly better outcome compared with patients with low MC counts.20 Instead, the correlation between MCs and outcome seems to be opposite in Hodgkin lymphoma.21 In FL, the role of MCs has not previously been investigated. The aim of our study was to identify whether MC content in the FL tissue has prognostic impact for the patients treated with combination of R and CHOP. Our data on immunochemotherapy-treated patients with FL demonstrate that tumor-infiltrating MCs have a significant negative impact on PFS. The effect on prognosis appears to be independent of FLIPI and TAMs, and is observed both for primary and relapsed patients. The finding that high MC counts predict unfavorable PFS after combination of R with chemotherapy at relapse but not after initial chemotherapy in the same patient cohort further suggests that addition of R to chemotherapy has a strong influence on the prognostic effect of MCs in FL.
As the first approach, we performed enzymatic Leder staining to identify MCs in the FL tissue. In our material, Leder stain–positive MC content predicted outcome both as a continuous and stratified variable. The finding is important since the cutoff levels best discriminating the low and high subgroups of biologic factors with prognostic significance vary considerably between different series. To validate the data, we used immunohistochemical detection of MC tryptase, and found a significant correlation between differently measured MC counts. This together with the finding that tryptase-positive MC counts also had a negative prognostic impact on PFS encourages us to believe that MC-based prognostic evaluations could be used as a tool to predict outcome of R-CHOP–treated patients with FL in the future. Nonetheless, it must be noted that the relative number of MCs was higher in tryptase-positive than in Leder-positive samples. Considering that tryptase stainings were performed on whole-tissue sections and Leder stainings primarily on TMAs, and that the relatively small size of TMA cores may hamper the reliable evaluation of many representative fields per sample, the discrepancy may be explained by this methodologic difference.
The mechanism by which the high MC counts disturb the outcome of immunochemotherapy-treated patients with FL is currently unknown. The finding that the prognostic impact of TAMs is lost in patients with many MCs suggests that MCs negatively regulate macrophage activity. Upon activation MCs secrete various biologically active factors, including growth factors and proteases that could be beneficial to tumor but harmful to immune system and antibody-based therapy.22 MCs also express Fcγ receptors, which can engage R.23 Considering that therapeutic effectiveness of R is dependent on Fc receptor–mediated interactions with other effector cells, including macrophages,24 it is plausible to suggest that high MC numbers negatively regulate antibody-dependent cellular cytotoxicity.
In conclusion, we have demonstrated that high MC score is associated with unfavorable prognosis in patients with FL treated with immunochemotherapy, and further observed that high MC count eliminates the prognostic effect of TAMs on survival. Clearly, the results should be confirmed prospectively in an independent cohort of immunochemotherapy-treated patients with FL. Nevertheless, they illustrate that the tumor microenvironment and host imflammatory response have a strong prognostic effect on survival of patients with FL in the post-R era of lymphoma therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Onerva Levälampi for technical assistance and the Molecular Imaging Unit (MIU) at the University of Helsinki for providing instruments and support with microscopy.
The study was supported by grants from the Finnish Medical Foundation, Finnish Cancer Societies, University of Helsinki, Juselius Foundation, and Helsinki University Central Hospital.
Authorship
Contribution: M.T. performed and scored histochemical stainings, analyzed the data, and assisted in writing; M.-L.K.-L. was responsible for verifying histology and analyzing histochemical stainings; and S.L. designed and supervised the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sirpa Leppä, Department of Oncology, Helsinki University Central Hospital, PO Box 180, FIN-00029 Helsinki, Finland; e-mail: sirpa.leppa@helsinki.fi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal