Abstract
Because of the low proliferative potential of tumor cells in patients with Sézary syndrome (SzS), their accumulation has been suggested to be due to defective regulation of apoptosis. We analyzed the sensitivity to soluble Fas-ligand (FasL) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), 2 members of the TNF superfamily in peripheral blood leukocytes (PBL) from patients with SzS. Compared with healthy donors, CD4+ cells from patients with SzS were completely resistant to FasL in 9 of 16 cases. Of these 9 FasL-resistant cases, 4 revealed a loss in Fas (CD95) expression, whereas the remaining 5 exhibited normal or enhanced Fas expression. In the latter 5 cases, the apoptosis inhibitor cFLIP was overexpressed in CD4+/CD26− tumor cells compared with CD4+/CD26− cells from Fas-expressing FasL-sensitive patients and healthy donors. Furthermore, resistance to TRAIL and tumor cell-restricted loss of TRAIL-receptor 2 were observed in 16 of 16 SzS PBLs. It is noteworthy that resistance to FasL could be overcome by the use of a hexameric FasL or upon exposure of SzS cells to interferon-α (IFN-α) or IFN-γ, the latter by an increase of Fas expression. Our data on primary SzS lymphocytes reveal frequent resistance to apoptosis induced by FasL and TRAIL, which may contribute to their accumulation in patients with SzS and be relevant at a therapeutic level.
Introduction
Sézary syndrome (SzS) is a leukemic variant of mycosis fungoides, a cutaneous T-cell lymphoma showing distinct clinical, histologic, immunologic, and genotypic features. SzS is characterized by the clonal proliferation of skin-invasive CD4+ T lymphocytes that have the phenotype of mature helper T cells.1 Patients with SzS manifest erythroderma, generalized lymphadenopathy, and prominent immunologic defects as a result of the production of immunosuppressive T-helper 2 (Th2) cytokines and by the depressed production of Th1 cytokines.1-3 The molecular cause of this Th2 shift remains to be identified, but its identification is likely to provide new therapeutic possibilities, because biologic response modifiers such as interferon-α (IFN-α), IFN-γ, and interleukin-12 (IL-12), which stimulate Th1 responses, have significant therapeutic effects in SzS.4,5
Apoptosis can be triggered by a series of stimuli, among which are signals generated by a family of transmembrane proteins—called death receptors—that belong to the tumor necrosis factor (TNF)-family of receptors. Six death receptors, namely Fas (CD95, Apo-1), TRAIL-receptor 1 (DR4), 2 (DR5, Apo-2), TNF-receptor 1, TRAMP (DR3, WSL-1, Apo-3), and DR6 have been identified,6,7 and all contain a cytoplasmic sequence named “death domain” that couples each receptor to caspase cascades essential for the induction of apoptosis.
The acquisition of resistance to apoptosis as one of the mechanisms involved in tumorigenesis is well documented. In many tumors, a decrease if not a loss of the expression of Fas compared with nontumor counterparts has been reported. In addition, mutations of Fas, TRAIL-R1, and TRAIL-R2 have been reported in several human cancers.8-14 In addition to an impaired expression of death receptors, aberrant expression of intracellular apoptosis inhibitors has been reported in many cancers. Among them, FLICE-inhibitory protein (cFLIP), which is highly homologous to caspases 8 and 10, contains a death effector domain through which it can bind to FADD and is able to block the autoproteolytic cleavage of caspase-8. An overexpression of cFLIP associated with a high expression of Fas has been reported in FasL-resistant Hodgkin/Reed-Sternberg cells, the malignant cells of classic Hodgkin lymphoma.15 An overexpression of cFLIP in ovarian cancer cells has been shown to be also associated with a resistance to cisplatin-induced apoptosis in vitro.16 Similar observations were also reported for non-Hodgkin lymphoma,17 bladder urothelial carcinoma,18 pancreatic cancer, stomach cancer,19 and melanoma.20 Finally, mouse models of lymphoma well document evidence for a role of cFLIP overexpression in tumor progression.21,22 Because of the low replicative potential of Sézary cells, their accumulation may to some extent be due to a defect in apoptosis pathways. Apoptosis defects in CTCL cell lines have recently been reported. These defects have been shown to affect the formation of the death-inducing signaling complex, including altered expression of death receptors, lack of caspase-8 activation, and cFLIP overexpression.23 The same study also reported an important expression of cFLIP in MF biopsies. However, the ex vivo sensitivity of primary circulating SzS cells to death-inducing ligands from the TNF family has never been reported to date. In the present work, we analyzed the sensitivity of primary ex vivo cultured circulating tumor cells from 16 patients with SzS to soluble FasL and TRAIL-mediated apoptosis, as well as the expression of selected molecules involved in their signaling.
Methods
Patient material and cell lines
Peripheral blood leukocytes (PBL) from patients with SzS were obtained after Ficoll isolation and frozen in fetal calf serum and 10% dimethyl sulfoxide until use. All the experiments described in the present work were performed with frozen PBL from patients with SzS diagnosed accordingly to ISCL criteria. Tumor burden (reported in Table 1) is defined as the percentage of circulating SzS cells and determined on sections of formalin-fixed peripheral blood buffy coats. T-cell receptor (TCR) rearrangement was positive for all the patients with SzS included in the present study. CD4/CD8 ratios are reported for each patient in Table 1. Donation of blood by patients or healthy volunteers in this study conformed to University of Pennsylvania institutional review board-approved protocol, and informed consents were obtained in accordance with the Declaration of Helsinki. The SzS cell line HUT78 was from the American Type Culture Collection (Manassas, VA). The MF cell line MyLa and the SzS cell line SeAx were kindly given by Prof K. Kaltoft and Prof H. Bachelez (Inserm, Hôpital Saint-Louis, Paris, France).
Characteristics of the SzS patients included in the study
| . | Patient no. . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | |
| Percentage SzS cells | 90 | 99 | 82 | 99 | 25 | 86 | 85 | 90 | 91 | 18 | 20 | 15 | 49 | 91 | 23 | 21 |
| CD4/CD8 ratio | 30 | 707 | 42 | 288 | 2 | 210 | 90 | 22.8 | 95 | 2.3 | 9.3 | 8 | ND | 115 | 9 | 40 |
| FasL sensitivity | – | – | – | – | – | – | – | – | – | + | + | + | + | + | + | + |
| Fas expression | – | – | + | + | + | – | + | – | + | + | + | + | + | + | + | + |
| TRAIL sensitivity | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| DR5 expression | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| cFLIP expression | ND | ND | ++ | ++ | ++ | ND | ++ | ND | ++ | ND | + | ND | ND | + | + | + |
| . | Patient no. . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | |
| Percentage SzS cells | 90 | 99 | 82 | 99 | 25 | 86 | 85 | 90 | 91 | 18 | 20 | 15 | 49 | 91 | 23 | 21 |
| CD4/CD8 ratio | 30 | 707 | 42 | 288 | 2 | 210 | 90 | 22.8 | 95 | 2.3 | 9.3 | 8 | ND | 115 | 9 | 40 |
| FasL sensitivity | – | – | – | – | – | – | – | – | – | + | + | + | + | + | + | + |
| Fas expression | – | – | + | + | + | – | + | – | + | + | + | + | + | + | + | + |
| TRAIL sensitivity | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| DR5 expression | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| cFLIP expression | ND | ND | ++ | ++ | ++ | ND | ++ | ND | ++ | ND | + | ND | ND | + | + | + |
Percentage of SzS cells was determined by flow cytometry or immunostaining of CD4+/CD26− cells in peripheral blood after cytospin. Ratios are calculated as follows: number of CD8/mL divided by number of CD4/mL of blood. CD4+ cells from patients with SzS were considered sensitive to FasL for <60% annexin-V-/PI-CD4+ cells by flow cytometry. Fas was considered to be significantly expressed when the FasΔMFI was >2 in CD4+ SzS cells.
ND indicates not determined; –, negative; +, positive/moderate; and ++, positive/high.
Sorting of CD4+/CD7− or CD4+/CD26− tumor cells
After thawing, CD4+/CD7− or CD4+/CD26− cells from patients with SzS and healthy donors (HD) were magnetically sorted using depletion of CD7+ or CD26+ cells followed by the positive selection of CD4+ cells with an AutoMacs (Miltenyi Biotech, Bergisch Gladbach, Germany). For the depletion of CD7+ or CD26+ cells, total PBL were stained with a phycoerythrin-conjugated anti-CD7 (clone M-T701; BD Biosciences, San Jose, CA) or anti-CD26 antibody (clone M-A261) and anti-phycoerythrin-conjugated beads (Miltenyi Biotech). The CD26− fraction was collected, labeled with anti-CD4-conjugated beads, and the CD4+ fraction was collected.
Analysis of the expression of death receptors
PBL from patients with SzS and HD were stained with monoclonal antibodies to CD4 (clone RPA-T4) and CD7 (clone M-T701) or CD26 (clone M-A261; all from BD Pharmingen), and monoclonal antibodies to Fas, TRAIL-receptor 1 (DR4, clone HS101), 2 (DR5, clone HS201), 3 (DcR1, clone HS301), and 4 (DcR2, clone HS402; Apotech, Lausen, Switzerland). Flow cytometric analysis was performed on a FACScan with the CellQuest Software (BD Biosciences, Allschwil, Switzerland).
Induction and analysis of apoptosis
Fresh (CTCL cell lines) or thawed (SzS and HD) cells were incubated at 106/mL in 96-well plates in 200 μl of RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum and antibiotics. SzS and HD CD4+ cells or CTCL cell lines were treated with increasing doses of soluble recombinant FasL (SuperFasLigand; Apotech), MegaFasLigand (kindly provided by Apoxis S.A./Topotarget Switzerland, Lausanne, Switzerland) or soluble recombinant TRAIL (SuperKillerTRAIL; Apotech) for 6 to 8 hours. Early apoptosis of the treated cells was analyzed by flow cytometry after staining with annexin-V (BD Pharmingen) and propidium iodide (Sigma-Aldrich, St Louis, MO). When indicated, cells were treated overnight with 100 IU/mL recombinant IFN-α or IFN-γ (Peprotech, London, United Kingdom) before treatment with SuperFasLigand.
Quantitative reverse transcription polymerase chain reaction
Total RNA were extracted with a QIAgen Mini Kit upon manufacturer's instructions and subjected to DNase I (Qiagen, Basel, Switzerland) treatment. Two micrograms of RNA were converted into cDNA using SuperScript II (Qiagen). The expression of cFLIP in purified CD4+/CD26− (magnetic cell sorting; Miltenyi Biotech) cells was compared with 3 stably expressed housekeeping genes, namely HsEEF1A1, GusB, and ALAS. The primers used for cFLIP cDNA amplification were CCAGAAGTACAAGCAGTCTGTTCAA (forward) and TTGGATTGCTGCTTGGAGAA (reverse).
Statistical analysis
When indicated, unpaired Student t test was applied. Differences were considered significant for P values less than.05.
Results
Heterogeneous FasL sensitivity in cutaneous lymphoma cell lines and circulating CD4+ cells from patients with SzS
Upon analyzing the sensitivity of a large number of cell lines to FasL, we could show that 1 of 3 CTCL cell lines was resistant to apoptosis when exposed in vitro to a hexameric form of soluble FasL (SuperFasL) that has previously been shown to be a potent inducer of Fas-mediated apoptosis in Fas expressing cell lines (Figure 1C and data not shown). As recently shown by Braun et al,23 we observed, with the use of a different apoptosis assay, that in the FasL-resistant SeAx cell line, the loss of sensitivity to soluble FasL correlated with a loss of Fas surface expression compared with the 2 other cell lines (HUT78 and MyLa; Figure 1C).
Heterogeneous Fas expression and loss of FasL sensitivity in SzS patient CD4+ cells. (A) CD4+ cells from HD (n = 10) and patients with SzS (n = 16) were subjected to FasL-induced apoptosis. Six to 8 hours after FasL exposure (100 ng/mL), cell viability was assessed by flow cytometry according to annexin-V and PI staining (top panel). CD4+ cells from healthy donors (HD, n = 10,  ) and patients with SzS (n = 16, ■) were stained with a fluorescein isothiocyanate (FITC)–anti-Fas (CD95) antibody or a FITC-isotype-matched control and analyzed by flow cytometry. ΔMFI expresses the ratio between the MFI of cells stained with the anti-Fas and the MFI obtained with cells stained with the isotype control antibody (bottom panel). Right panels show representative Fas expression patterns (CD95 antibody, bold histogram; control isotype, thin histogram) in selected individuals. (B) Correlation between FasL-induced apoptosis and tumor burden in patients with SzS. (C) Sensitivity to FasL and Fas expression (100 ng/mL) in the CTCL cell lines HUT78 (SzS), MyLa (MF), and SeAx (SzS).
) and patients with SzS (n = 16, ■) were stained with a fluorescein isothiocyanate (FITC)–anti-Fas (CD95) antibody or a FITC-isotype-matched control and analyzed by flow cytometry. ΔMFI expresses the ratio between the MFI of cells stained with the anti-Fas and the MFI obtained with cells stained with the isotype control antibody (bottom panel). Right panels show representative Fas expression patterns (CD95 antibody, bold histogram; control isotype, thin histogram) in selected individuals. (B) Correlation between FasL-induced apoptosis and tumor burden in patients with SzS. (C) Sensitivity to FasL and Fas expression (100 ng/mL) in the CTCL cell lines HUT78 (SzS), MyLa (MF), and SeAx (SzS).
Heterogeneous Fas expression and loss of FasL sensitivity in SzS patient CD4+ cells. (A) CD4+ cells from HD (n = 10) and patients with SzS (n = 16) were subjected to FasL-induced apoptosis. Six to 8 hours after FasL exposure (100 ng/mL), cell viability was assessed by flow cytometry according to annexin-V and PI staining (top panel). CD4+ cells from healthy donors (HD, n = 10,  ) and patients with SzS (n = 16, ■) were stained with a fluorescein isothiocyanate (FITC)–anti-Fas (CD95) antibody or a FITC-isotype-matched control and analyzed by flow cytometry. ΔMFI expresses the ratio between the MFI of cells stained with the anti-Fas and the MFI obtained with cells stained with the isotype control antibody (bottom panel). Right panels show representative Fas expression patterns (CD95 antibody, bold histogram; control isotype, thin histogram) in selected individuals. (B) Correlation between FasL-induced apoptosis and tumor burden in patients with SzS. (C) Sensitivity to FasL and Fas expression (100 ng/mL) in the CTCL cell lines HUT78 (SzS), MyLa (MF), and SeAx (SzS).
) and patients with SzS (n = 16, ■) were stained with a fluorescein isothiocyanate (FITC)–anti-Fas (CD95) antibody or a FITC-isotype-matched control and analyzed by flow cytometry. ΔMFI expresses the ratio between the MFI of cells stained with the anti-Fas and the MFI obtained with cells stained with the isotype control antibody (bottom panel). Right panels show representative Fas expression patterns (CD95 antibody, bold histogram; control isotype, thin histogram) in selected individuals. (B) Correlation between FasL-induced apoptosis and tumor burden in patients with SzS. (C) Sensitivity to FasL and Fas expression (100 ng/mL) in the CTCL cell lines HUT78 (SzS), MyLa (MF), and SeAx (SzS).
To understand the cause of the above observed resistance to soluble FasL in CD4+ cells from patients with SzS, we analyzed the expression of Fas on CD4+ cells from HD and patients with SzS. In HD, Fas expression on CD4+ cells was quite strong and varied little from donor to donor (Δmean fluorescence intensity [MFI] = 4.9 ± 1.09) (Figure 1A). This was not the case in patients with SzS, in which Fas expression on CD4+ cells varied considerably among patients. Four of 16 (25%) patients with SzS (patients 1, 2, 6, and 8), exhibited a decreased level of Fas expression on CD4+ (mean MFI = 1.7) cells compared with HD (mean MFI = 4.75). The remaining 12 of 16 patients (75%) revealed similar (patients 3, 4, 7, 11, 13, 15 and 16; mean MFI = 5) or higher (patients 5, 9, 10, 12 and 14; mean MFI = 8.2) levels of Fas expression on CD4+ cells compared with HD. Because tumor cells comprise only a fraction of the total CD4+ cells, analysis of Fas expression was also specifically analyzed on CD4+/CD7−/CD26− cells that are known to be the phenotypic markers for tumor cells in patients with SzS. In both HD and SzS CD4+ cells, no significant difference in Fas expression was observed between the CD26+/CD7+ and the CD26−/CD7− subpopulations, indicating that the observed loss of Fas expression on CD4+ cells in patients with SzS is not selective for the tumor population of CD4+ cells (data not shown).
To analyze the sensitivity of circulating CD4+ cells from patients with SzS and HD to FasL, fresh or thawed PBL were exposed in vitro to soluble FasL (SuperFasL), and apoptosis was assessed by annexin-V/PI staining. In the 10 HD tested, FasL induced apoptosis of CD4+ cells in a dose-dependent manner, with an average of 50% apoptosis (46.5 ± 6.5% [SD]) occurring after 8-hour exposure to 100 ng/mL of soluble FasL (Figure 1A). In patients with SzS CD4+ cells, the degree of apoptosis induced by soluble FasL under the same conditions varied significantly among patients. Within the 16 patients with SzS tested, CD4+ cells from 9 of 16 patients with SzS (56%, patients 1-9) were significantly (P < .05) more resistant to soluble FasL-mediated apoptosis (90 ± 6.9% viable cells) than HD CD4+ cells (Figure 1A, Table 1). In the remaining 7 patients with SzS (44%, patients 10-16) sensitivity to FasL was similar to that of HD (mean 46.8 ± 11.3% apoptotic cells) (Figure 1A and Table 1). Furthermore, a certain degree of correlation (R2 = 0.54) between FasL-resistance and tumor burden was observed (Figure 1B). It is noteworthy that in patients with a high tumor burden (> 80% circulating SzS cells) FasL was not able to induce apoptosis of CD4+ cells (89.5% ± 15% viable CD4+ cells), whereas an average of 50% CD4+ cell apoptosis (51.6 ± 17.3%) was observed in patients with low or medium (< 50% circulating SzS cells) tumor burden (Figure 1B, Table 1).
In 4 of 16 (25%) patients with SzS, the significantly reduced Fas expression on CD4+ cells was correlated with significant resistance to FasL-induced apoptosis (patients 1, 2, 6, and 8; Figure 1A). In 7 of 16 (43.75%) patients with SzS, CD4+ Fas expression correlated well with sensitivity to FasL-induced apoptosis (patients 10-16). Finally, in the remaining 5 of 16 (31.25%) patients with SzS, significant resistance to FasL-induced apoptosis was observed despite CD4+ cell Fas expression levels similar or higher than that observed in HD (patients 3, 4, 5, 7, and 9). The above observations indicate that decreased Fas expression on SzS CD4+ cells was not the sole mechanism responsible for resistance to soluble FasL.
Overexpression of the cFLIP gene in circulating tumor cells from patients with SzS
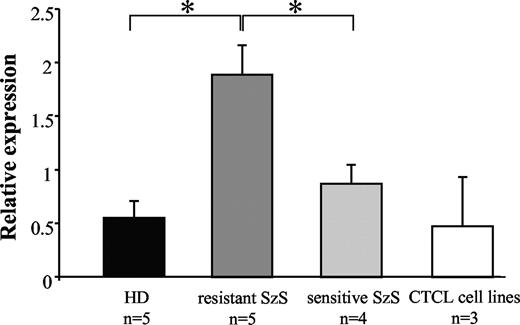
The observation that 5 of 16 patients with SzS (patients 3, 4, 5, 7, and 9) CD4+ cells were resistant to soluble FasL-induced apoptosis, despite significant Fas expression, suggests that molecular mechanisms other than loss of Fas-expression may account for the observed apoptosis resistance. To assess whether the intracellular death receptor-mediated apoptosis inhibitor cFLIP could be involved in apoptosis resistance, quantitative cFLIP real-time polymerase chain reaction (RT-PCR) was performed on sorted CD4+/CD26− cells from the 5 patients described above and compared with Fas-expressing FasL-sensitive patients with SzS (n = 4, patients 11, 14, 15, and 16) and HD (n = 5). The magnetically sorted CD4+/CD26− populations exhibited a purity of more than 95% (data not shown). In the 5 tested CD4+/CD26− populations from FasL-resistant patients with SzS, the relative expression of cFLIP was significantly increased compared with CD4+/CD26− cells from FasL-sensitive patients with SzS (1.9 ± 0.26 vs 0.8 ± 0.17, respectively; P < .05; Figure 2, Table 1) and HD (1.9 ± 0.26 vs 0.45 ± 0.15, respectively; P < .05; Figure 2, Table 1). The same analysis was performed in the HUT78, MyLa, and SeAx CTCL cell lines, and cFLIP gene expression was found to be similar to that measured in healthy donors (0.47 ± 0.45 vs 0.45 ± 0.17, respectively; P = not significant; Figure 2). These data suggest that cFLIP overexpression may be associated with resistance to FasL-mediated apoptosis in Fas-expressing CD4+ cells in patients with SzS.
cFLIP is overexpressed in SzS CD4+/CD26− cells. cDNA from HD (n = 5), Fas-expressing FasL-resistant SzS (n = 5, patients 3, 4, 5, 7, and 9), Fas-expressing FasL-sensitive (n = 4, patients 11, 14, 15, and 16) CD4+/CD26− sorted cells and HUT78, MyLa, and SeAx cell lines were amplified using cFLIP specific primers and compared with HsEEF1A1, GusB, and ALAS expression. Mean (± SD) of the relative expression is reported.
cFLIP is overexpressed in SzS CD4+/CD26− cells. cDNA from HD (n = 5), Fas-expressing FasL-resistant SzS (n = 5, patients 3, 4, 5, 7, and 9), Fas-expressing FasL-sensitive (n = 4, patients 11, 14, 15, and 16) CD4+/CD26− sorted cells and HUT78, MyLa, and SeAx cell lines were amplified using cFLIP specific primers and compared with HsEEF1A1, GusB, and ALAS expression. Mean (± SD) of the relative expression is reported.
CD4+ cells from patients with SzS are resistant to TRAIL, and the CD4+/26− tumor cell population selectively lacks surface TRAIL-R2 (DR5)
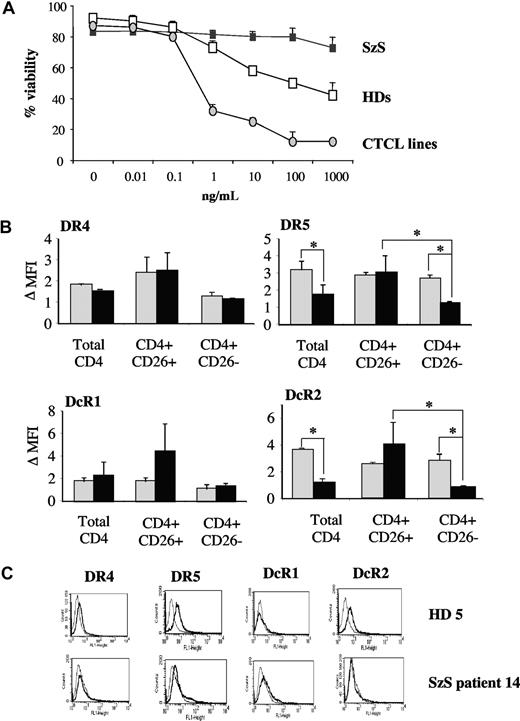
Because soluble TRAIL is in clinical development for the treatment of cancer, we tested the sensitivity of CD4+ cells from HD and patients with SzS to soluble TRAIL. In contrast to CTCL cell lines and, to a lesser extent HD CD4+ cells, which are both shown to be sensitive to soluble TRAIL in a dose-dependent manner, CD4+ cells from all 16 (100%) tested patients with SzS were completely resistant to TRAIL-induced apoptosis in vitro (Figure 3A, Table 1). Furthermore, the observed resistance to TRAIL-induced apoptosis in patients with SzS was independent of tumor burden (Table 1).
Tumor-restricted loss of DR5 and TRAIL resistance in SzS patient CD4+ cells. (A) Cells from HD, patients with SzS and CTCL cell lines were exposed to increasing doses of soluble TRAIL. Viability was determined upon annexin-V/PI staining. One representative of 3 experiments. (B) Mean (± SD) expression of TRAIL-receptors in CD4+ cells from healthy donors (gray histogram, n = 10) and patients with SzS (black histogram, n = 16). *P < .05. (C) Representative TRAIL-receptor expression profile in HD and patients with SzS.
Tumor-restricted loss of DR5 and TRAIL resistance in SzS patient CD4+ cells. (A) Cells from HD, patients with SzS and CTCL cell lines were exposed to increasing doses of soluble TRAIL. Viability was determined upon annexin-V/PI staining. One representative of 3 experiments. (B) Mean (± SD) expression of TRAIL-receptors in CD4+ cells from healthy donors (gray histogram, n = 10) and patients with SzS (black histogram, n = 16). *P < .05. (C) Representative TRAIL-receptor expression profile in HD and patients with SzS.
To further investigate the cause of resistance to TRAIL, the expression of the death receptors TRAIL-receptors 1 (DR4) and 2 (DR5) and the decoy receptors TRAIL receptors 3 (DcR1) and 4 (DcR2) was investigated by fluorescence-activated cell sorting in PBL from 10 HD and 16 patients with SzS. No difference of expression of DR4 and DcR1 was found between HD and patients with SzS on total CD4+ cells or CD4+ CD26− cells (Figure 3B,C). By contrast, the expression of both TRAIL-receptor 2 (DR5) and DcR2 were found to be significantly decreased in total CD4+ cells from patients with SzS compared with HD (Figure 3B, Table 1). It is noteworthy that the observed loss in TRAIL-receptor 2 (DR5) and DcR2 expression selectively affected the CD4+/CD26− cells from patients with SzS (Figure 3B). Taken together, these data reveal a selective loss of TRAIL-receptor 2 (DR5), the major receptor involved in TRAIL-induced apoptosis, in a tumor-restricted manner.
A novel hexameric form of FasL (MegaFasL) or preincubation with IFN-α/γ could partially overcome the resistance of SzS CD4+ cells to apoptosis
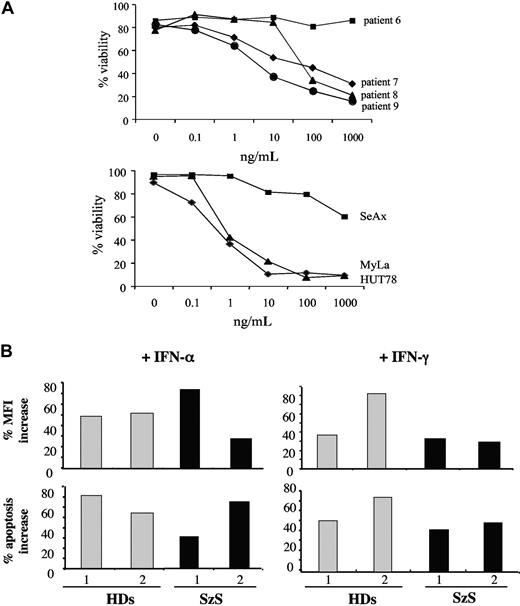
CD4+ cells from patients resistant to apoptosis induced by the above agents as well as commercially available soluble FasL (SuperFasL) were exposed to a novel genetically engineered hexameric form of soluble FasL (megaFasL [MFL]). For this experiment, 4 FasL-resistant patients were selected (patients 6, 7, 8, and 9; see Figure 1A, Table 1). By contrast to SuperFasL, MFL was able to induce significant apoptosis in circulating tumor cells from 3 of 4 (75%) of the selected patients (patients 7, 8, and 9). In these patients, the average IC50 after 8 hours in vitro MFL exposure ranged from 3 to 50 ng/mL, as opposed to more than 1 μg/mL with SuperFasL. No apoptosis was observed in patient 6 in whom Fas expression on CD4+ cells was completely absent (Figure 4).
Resistance to apoptosis induced by soluble FasL can be overcome in patients with SzS. (A) Selected resistant patients expressing low levels (patients 7, 8, and 9) or no Fas (patient 6) were exposed to increasing doses of MFL. Viability was assessed 6 to 8 hours after MFL treatment by flow cytometry analysis of annexin-V/PI staining. One representative of 2 experiments. In the same way, Fas-expressing HUT78 and MyLa and Fas-negative SeAx cell lines were treated with MFL. (B) Two selected HD (1 and 2) and patients with SzS (1 and 2) were exposed to 100 IU/mL IFN-α (left panels) or IFN-γ (right panels) before treatment with 100 ng/mL FasL. Increase of the measured Fas-MFI (top panels) and increase of the annexin-V+/PI− apoptotic fraction (bottom panels) are reported.
Resistance to apoptosis induced by soluble FasL can be overcome in patients with SzS. (A) Selected resistant patients expressing low levels (patients 7, 8, and 9) or no Fas (patient 6) were exposed to increasing doses of MFL. Viability was assessed 6 to 8 hours after MFL treatment by flow cytometry analysis of annexin-V/PI staining. One representative of 2 experiments. In the same way, Fas-expressing HUT78 and MyLa and Fas-negative SeAx cell lines were treated with MFL. (B) Two selected HD (1 and 2) and patients with SzS (1 and 2) were exposed to 100 IU/mL IFN-α (left panels) or IFN-γ (right panels) before treatment with 100 ng/mL FasL. Increase of the measured Fas-MFI (top panels) and increase of the annexin-V+/PI− apoptotic fraction (bottom panels) are reported.
IFN-α has proven clinical efficacy in the treatment of CTCL including SzS. To determine whether IFN can affect the sensitivity to FasL, CD4+ cells from 2 selected patients with SzS with low Fas expression (patients 1 and 2) and from 2 healthy donors (HD 1 and 2) were exposed in vitro overnight to 100 IU/mL IFN-α or IFN-γ and subsequently exposed to 100 ng/mL of SuperFasL. Exposure to either IFN-α or IFN-γ resulted in a 40% to 70% increase in Fas expression on the surface of CD4+ cells in 2 of 2 HD and 2 of 2 patients with SzS (Figure 4B top panels). When apoptosis was assessed under the same conditions, the observed increased Fas expression upon exposure to IFN-α or IFN-γ was associated with a significant (40%-80%) increase in FasL-mediated apoptosis (Figure 4B bottom panels).
Discussion
In the present work, we demonstrate that in patients with SzS, CD4+ T cells harbor significant defects in death receptor-mediated apoptosis signaling. These defects affect both the FasL and TRAIL signaling pathways and are associated in all cases with either a loss of expression of the cell surface receptor for these 2 ligands, or an increased expression of the gene encoding the intracellular apoptosis inhibitor cFLIP. Furthermore, we show that in the case of Fas, at least, this signaling defect can be circumvented in a potentially therapeutically meaningful manner in vitro either with a genetically engineered hexameric form of FasL or by pre-exposure to IFN-α or IFN-γ.
Appropriate Fas-mediated cell death is essential for adequate control of lymphocyte homeostasis. It is noteworthy that elimination of T cells after encountering a specific antigen or superantigen proceeds via activation-induced cell death, a process that is dependent on appropriate Fas signaling of cell death. Furthermore, in both mice and humans, Fas gene mutations leading to defective Fas/signaling result in lymphoproliferative disorders as a consequence of lymphocyte accumulation.24,25 Because in CTCL, tumor cells have been demonstrated to be long-lived activated CD4+ cells, defects in Fas-mediated apoptosis have been previously proposed based on studies of protein expression, but the functional consequences and detailed analyses of Fas signaling in CTCL is lacking.26 It has recently been shown that apoptosis defects exist in CTCL cells, but the majority of the reported functional data were obtained with 4 established CTCL cell lines, a setting that does not necessarily adequately reflect the clinical situation.23 In the present work, we specifically analyzed apoptosis defects in ex vivo primary SzS cells from 16 patients.
Using immunohistochemistry, decreased Fas expression by semiquantitative analysis of anti-Fas labeling of skin-infiltrating CD4+ cells has been reported in tumor-stage MF and CD30− large cutaneous large T-cell lymphoma.26 Likewise, in 4 and 3 cases of SzS, Dereure et al27 and Meech et al,28 respectively, provide data showing that Fas expression is decreased on circulating CD4 or CD45RO-positive cells without further specification of the T-cell phenotype.27,28 In the present work on a significant number of patients with SzS, we functionally demonstrate for the first time that, compared with healthy donors, circulating CD4+ cells from patients with SzS are completely resistant to soluble FasL in 56% of cases. It is noteworthy that this functional defect in Fas signaling is only accounted for by a loss of Fas expression in 44% of the Fas-resistant cases, and in such cases, the loss of Fas expression was not restricted to CD4+/CD26− cells phenotypically consistent with the tumor cell population. The cause of the observed loss of Fas expression on tumoral and nontumoral CD4+ T cells of a subset of patients with SzS remains to be determined. Fas gene mutations or splice variants have been reported in MF but no data are available for SzS; moreover, the latter would not account for the observed loss of Fas expression on nontumoral CD4+ T cells of patients with SzS.29,30 A possible explanation that remains to be investigated is that the Th2 cytokine milieu that predominates in SzS31 may affect Fas expression and/or susceptibility to Fas-mediated apoptosis in CD4+ cells. In support of this, in a recent report, Zhang et al32 show that Th2 cells are more resistant to either TRAIL- or FasL-mediated apoptosis than Th1 cells. Likewise, the Th2 cytokine IL-4 has been shown to protect tumor cells from FasL-mediated apoptosis.33
Among genes that have been suggested to be modulated by Th2 cytokines is cFLIP, a cytoplasmic protein highly homologous to caspases 8 and 10 that is a potent inhibitor of death-receptor mediated apoptosis. The Th2 cytokine IL-4 has been shown to protect solid tumor cells from FasL-mediated apoptosis via up-regulation of antiapoptotic proteins including FLIP.33 cFLIP has been shown to be strongly expressed in skin biopsies of MF patients by immunohistochemistry. The same study reported cFLIP expression in the PBL of patients with SzS without specifically looking at CD4+/CD26− and at levels and frequency not distinct from that found in healthy donor PBL.23 In the present work, we quantified cFLIP expression in sorted CD4+/CD26− tumor cells from the 5 FasL-mediated apoptosis-resistant, Fas-expressing patients with SzS. cFLIP overexpression was found in the 5 tested CD4+/CD26− cells from Fas-expressing FasL-resistant patients with SzS, irrespective of the tumor burden. Such an overexpression was not observed in CD4+/CD26− cells from FasL-sensitive patients with SzS, healthy donors, and CTCL cell lines, suggesting that, as reported for other cancers, increased expression of cFLIP may contribute to defective death-receptor signaling. Whether Th2 cytokines such as IL-4 are responsible for the high levels of cFLIP mRNA observed selectively in the CD4+/CD26− cell population of patients with SzS remains to be determined. Nevertheless, the observed increased cFLIP expression in SzS is likely to be of relevance in disease pathogenesis as suggested in mouse models of lymphoma in which enforced overexpression of cFLIP has been shown to favor tumor progression.21,22 In their recent publication, Braun et al23 show an increased expression of cFLIP in CTCL cell lines compared with acute lymphoid leukemia cell lines. We also found such an increased expression in CTCL cell lines and primary tumor cells from patients with SzS. However, unlike the Western blot data of Braun et al,23 we found, using qRT-PCR, significant differences in cFLIP expression between FasL-resistant CD4+/CD26− cells from patients with SzS and CD4+/CD26− cells from HD, suggesting that this overexpression may directly contribute to Fas resistance despite conserved Fas expression in patients with SzS.
The death ligand TRAIL is considered to be a promising anticancer agent. Agonistic antibodies against DR4,34 or DR5,35 or recombinant soluble TRAIL36-39 are able to induce apoptosis in cancer cells of various origins in vitro and in vivo without affecting normal cells. TRAIL is currently being evaluated clinically in a phase Ib as an anticancer agent in a variety of solid tumors and hematologic malignancies. Gene expression profiling by qRT-PCR showed an overexpression of TRAIL in progressive CTCL,40 but the expression of TRAIL receptors and sensitivity of SzS primary tumor cells to TRAIL has not been reported to date. Here, we provide novel data revealing that circulating CD4+ cells from patients with SzS are completely resistant to soluble TRAIL and such patients harbor a significant, tumor cell–restricted, loss of TRAIL-receptor 2 (DR5). It is noteworthy that DR5 was expressed by CD4+/CD26+ nontumor cells from patients with SzS, thus suggesting that the total resistance to TRAIL is most likely a tumor-associated event. Loss or altered expression of DR5 has been reported previously for certain other cancers.10,13 This data suggests that the use of TRAIL as single anticancer agent may be ineffective in SzS.
In the present work, we have investigated 2 approaches to circumvent the observed resistance of PBL from patients with SzS to apoptosis mediated by Fas signaling. As previously shown for acute myeloid leukemia,41 the use of recently developed soluble hexameric form of FasL, namely MFL, allowed us to induce significant apoptotic cell death in all the tested SzS tumor cell samples. The reason of the increased efficiency of MFL compared with other forms of soluble FasL remains unknown. A possible increase of the recruitment of weakly expressed Fas at the surface of tumor cells by MFL due to its hexameric nature (compared with mono or trimers) or to its own conformation (compared with the SuperFasL hexamer used in this study) may be an explanation. It is noteworthy that the low observed IC50 of MFL on SzS PBL in vitro suggests that there may be a therapeutic window of opportunity for the uses of MFL in the treatment of SzS. Indeed, circulating levels of MFL that were 10 to 100 times higher than the concentrations necessary to kill tumor cells in vitro were attained after injection of MFL in mice, in the absence of severe systemic toxicity.42 Accordingly, MFL treatment of mice bearing xenograft tumors of ovarian cancer resulted in a decrease in tumor growth at tolerated doses. On the basis of these and other favorable results, MFL is currently being tested as an anti-tumor agent in phase I clinical trials.
The 2 main objectives of SzS treatment are (1) to favor the elimination of tumor cells and (2) to restore a normal immune response. Multimodality therapy, which is now the standard of care for advanced CTCL, includes the use of agents that induce apoptosis such as PUVA or, more recently, bexarotene, a retinoid X receptor specific agent.43 Combinations of apoptotic agents and agents targeting the activation of immune cells are known to induce higher therapeutic responses than apoptotic agents alone.44 As an example, treatment associating PUVA and IFN-α leads to a higher clinical response in CTCL patients than PUVA alone. Here we investigated the effect of IFN-α and IFN-γ on the sensitivity of patients with SzS PBL to soluble FasL and could demonstrate that pretreatment with either of these cytokines could increase Fas expression on SzS tumor cells and simultaneously increase their sensitivity to soluble FasL.
Taken together, our observations suggest that resistance to apoptosis induced by the TNF ligands FasL and TRAIL occur in a significant subset of patients with SzS. This may contribute to the observed accumulation of CD4+/CD26− clonal T cells in the disease and should be taken into account for the design of therapeutic strategies in SzS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Fonds National Suisse de la Recherche Scientifique (L.E.F.), Oncosuisse (L.E.F./E.C.), the Fondation Louis Jeantet and the EU Transeurope/Transnet, and a Translation Research grant from the Leukemia and Lymphoma Society (A.H.R.).
Authorship
Contribution: E.C., K.K., S.R., O.G., M.D., and R.S performed the experiments; A.H.R. and L.E.F. designed the research; E.C. and L.E.F. wrote the article.
Conflict-of-interest disclosure: L.E.F. declares a financial interest in Topo-target, a company whose potential product was studied in the present work. The remaining authors declare no competing financial interests.
Correspondence: Lars E. French, Department of Dermatology, Zurich University Hospital, Gloriastrasse 31, 8091 Zürich, Switzerland; e-mail: lars.french@usz.ch.
References
Author notes
E.C. and K.K. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal