Abstract
In B lymphocytes, the B-cell adaptor for phosphatidylinositol 3-kinase (BCAP) facilitates signaling from the antigen receptor. Mice lacking BCAP have a predominantly immature pool of B cells with impaired immune function and increased susceptibility to apoptosis. Unexpectedly, we have found that natural killer (NK) cells from BCAP-deficient mice are more mature, more long-lived, more resistant to apoptosis, and exhibit enhanced functional activity compared with NK cells from wild-type mice. Surprisingly, these effects are evident despite a severe impairment of the immunoreceptor tyrosine-based activation motif-mediated Akt signaling pathway. The seemingly paradoxical phenotype reveals inherent differences in the signals controlling the final maturation of B cells and NK cells, which depend on positive and negative signals, respectively. Both enhanced interferon-γ responses and augmented maturation of NK cells in BCAP-deficient mice are independent of available MHC class I ligands. Our data support a model in which blunting of BCAP-mediated activation signaling in developing NK cells promotes functionality, terminal maturation, and long-term survival.
Introduction
The expression of self-recognizing inhibitory receptors (SRIRs) that engage major histocompatibility complex class I (MHC-I) is an important step during natural killer (NK)–cell development. Expression of SRIRs tolerizes mature NK cells toward normal cells but allows the attack of MHC-I-deficient tumors or virus-infected cells, which is known as “missing self-recognition.”1
In mice, the Ly49 genes encode a polymorphic lectin-like receptor family consisting of both activating and inhibitory members that are expressed on NK cells and some T cells.2 Some activating Ly49 members can detect specific virus-encoded products, whereas individual inhibitory Ly49 members recognize distinct MHC-I allotypes and are important in self-recognition by NK cells.2 Multiple inhibitory Ly49 family members are stochastically expressed on individual mature NK cells, with most clones expressing one to 3 distinct gene products.3,4 This variegated receptor expression generates a complex repertoire of NK cells that appears to persist throughout the lifetime of the cells. Some inhibitory Ly49 genes expressed in an individual mouse produce receptors that cannot recognize the endogenous MHC-I. Both in vitro and in vivo experiments support a “regulated sequential” developmental model in which individual Ly49 receptors are randomly expressed during NK-cell development until one recognizes self MHC-I, transduces negative signals, and promotes full maturation, thereby restricting expression of additional Ly49 genes.5-10
Early evidence suggested that all mature NK cells in mice or humans possess at least one inhibitory receptor that recognizes the endogenous MHC-I.11,12 Recent work has challenged this concept, however, because a subset of NK cells has been identified that lacks self-recognition.13-16 Interestingly, this subset was found to be hyporesponsive, similar to NK cells derived from MHC-I–deficient mice.17
Phosphatidylinositol 3-kinase (PI3-K) is a key enzyme for NK- cell activation responses, particularly in cytolytic granule mobilization and resistance to apoptosis.18-20 The regulatory subunits of class IA PI3-K complexes contain 2 SH2 domains that specifically bind to tyrosine phosphorylated sites (pYxxM) on membrane-associated receptors or adaptors, thereby recruiting the enzyme near substrate.21 B-cell adaptor for PI3-K (BCAP) was identified as a cytosolic adaptor protein that can recruit PI3-K when tyrosine-phosphorylated on YxxM motifs.22 BCAP is expressed in B cells, macrophages, and dendritic cells, but not in T or mast cells. B cells from mice deficient in BCAP have an impaired ability to reach full maturity, produce less immunoglobulin, have decreased proliferative capability, are more susceptible to apoptosis, and exhibit reduced calcium mobilization in response to antigen receptor (BCR) crosslinking.23 BCAP-deficient B cells also display a selective deficit in expression of the NF-κB family member, c-Rel, and decreased expression of several NF-κB target genes that are essential for normal cell proliferation and survival.24 Little is known, however, about the molecular functions of BCAP, which has potential to serve as a scaffolding structure for docking many signaling proteins, including PI3-K.
Here we show, for the first time, that BCAP is strongly expressed in NK cells. More importantly, we demonstrate that BCAP plays an important role in NK-cell development that is very different from that previously observed in B cells. NK cells from BCAP-deficient mice exhibit enhanced cytolytic capability, produce more interferon-γ (IFN-γ), are more resistant to apoptosis, and more readily attain a fully mature state than NK cells from wild-type (WT) mice. The results provide important insights regarding the signals driving NK-cell maturation and responsiveness. The opposite phenotypes observed in B and NK cells from BCAP−/− mice also expose major differences in the terminal maturation checkpoints controlling development of these 2 lymphocyte lineages.
Methods
Mice
BCAP−/− mice were previously described.23 Mice were rederived at Fox Chase Cancer Center and maintained in a closed breeding facility. Mice were backcrossed a total of 7 generations onto a C57BL/6 background for these studies, and subsequent backcrossing to 9 generations revealed no change in phenotype. C57BL/6 mice were from The Jackson Laboratory (Bar Harbor, ME) or bred in house. KbDb−/− and B6.C-H2d/bByJ (H2d congenic) mice were purchased from Taconic Farms (Germantown, NY) and The Jackson Laboratory, respectively. The B6.C-H2d/bByJ mouse (H2d) is C57BL/6 except that H-2Db and H-2Kb loci contain H-2Dd and H-2Kd. These mice were crossed to BCAP−/− mice in house. Mice were housed, treated, infected, and killed in accordance with protocols approved by our Institutional Animal Care and Use Committee that follows Association for Assessment and Accreditation of Laboratory Animal Care regulations.
Cell preparation and flow cytometry
Splenocytes were mashed through a 40-μm nylon cell strainer and erythrocytes were lysed in ACK buffer (125 mM of NH4Cl, 10 mM of KHCO3, 1 mM of Na2EDTA) for 10 minutes on ice. NK1.1+CD3− NK cells were sorted on a BD FACSVantage-SE flow cytometer (BD Biosciences, San Jose, CA). Bone marrow cells were irrigated from femurs with RPMI 1640 medium, followed by erythrocyte lysis. For surface staining, cells were preincubated with unlabeled anti-CD16 antibody (clone 93; eBioscience, San Diego, CA) to prevent Fc receptor binding. Cells were analyzed on a BD LSR-II flow cytometer. Antibodies were purchased from BD Biosciences PharMingen (San Jose, CA), BioLegend (San Diego, CA), eBioscience, or Southern Biotechnology Associates (Birmingham, AL). Anti-BCAP (4L8E6) and anti-CD56 (B159) were protein G purified from hybridomas. Propidium iodide was used to exclude dead cells, except when permeabilized. The histograms are not peak normalized, but rather are drawn from sample sets with equal numbers of cells. NK cells are gated as NK1.1+, CD3−.
We originally assumed that the 5E6 antibody (BD Biosciences PharMingen) recognizes both Ly49C and Ly49I in C57BL/6 mice, as described by the manufacturer, and reported by Brennan et al.25 However, in our hands, 5E6 appears only capable of recognizing Ly49I on NK cells in these mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This may be the result of strong cis masking of Ly49C by H-2b on the NK-cell surface, which is less evident for Ly49I.26 Biotinylated anti-Ly49C 4LO3311 antibody was a generous gift from Dr Suzanne Lemieux at the Institute National de la Recherche Scientifique (INRS), University of Quebec, Canada. Throughout this manuscript, we refer to 4LO3311 as anti-Ly49C, and YLI-90 as anti-Ly49I.
Cytotoxicity
Cytotoxicity was measured with a 4-hour 51Cr-release assay.27 For CHO-K1 targets, the percentage of NK cells in fresh splenocytes was measured using flow cytometry, and stated ratios of NK to target cells were apportioned. Cytotoxicity against YAC-1 was measured as (1) above, but mice received intraperitoneal injections of polyriboinosinic:polyribocytidylic acid (poly I:C; 200 μg/500 μL of phosphate-buffered saline [PBS]) 2 days before or (2) NK cells were sorted (NK1.1+, CD3−) and cultured with 1000 U/mL recombinant human IL-2 (Roche, Basel, Switzerland; from the NCI Biologic Resources Branch, Frederick, MD) in complete RPMI 1640 (10% fetal bovine serum, 100 μg/mL penicillin/streptomycin, 2 mM l-glutamine, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol). Target cells were cultured in complete RPMI 1640 medium.
Ectromelia virus assays
A total of 3000 plaque-forming units of ectromelia virus in 50 μL PBS was injected into the back left footpads of mice, which were housed in a P3 facility for 5 or 7 days before the splenocytes were harvested and spread on BSC-1 cells in serial tenfold dilutions, then incubated for 5 days at 37°C in RPMI 1640 plus 2.5% fetal bovine serum. Plaques were resolved with a 0.1% crystal violet solution and counted.
Interferon-γ-producing cell assay
Fresh splenocytes were harvested, distributed into a 12-well plate (4 million per 2 mL complete RPMI 1640) that had been coated overnight with phycoerythrin (PE)- or biotin-conjugated anti-NK1.1 monoclonal antibody (mAb; 10 μg/mL PK136 in carbonate/bicarbonate buffer, pH 9.0) plus human rIL-2 (500 U/mL) and mouse rIL-12 (10 ng/mL). Cells were incubated at 37°C for 5 hours, with 10 μg/mL brefeldin A added after 1 hour, then removed from the plate with nonenzymatic cell dissociation solution (Sigma-Aldrich, St Louis, MO). The cells were surface stained as above, fixed with 4% paraformaldehyde, and permeabilized for 30 minutes at room temperature in PBS containing 0.1% saponin, 1 mM CaCl2, 1 mM MgSO4, 0.05% sodium azide, 1% BSA, and 10 mM HEPES (PBS-S; pH 7.4), then stained with 40 ng/mL Pacific Blue–conjugated anti-IFN-γ (eBioscience).
Akt phosphorylation assay
NK cells were sorted on a BD FACSVantage-SE flow cytometer and cultured for 8 days in complete RPMI 1640 with 1000 U/mL human r-IL-2, then 5 × 105 cells were counted into 0.5 mL of Hank balanced salt solution and stimulated at 37°C with 4 μg biotinylated anti-NK1.1 (eBioScience) followed 1 minute later by 8 μg streptavidin (Sigma-Aldrich). After the indicated time, the reaction was stopped with 1 mL ice-cold phospho-stop buffer (PBS, 1 μg microcystin, one Complete protease inhibitor tablet per 100 mL [Roche], 10 mM NaF, 400 μM EDTA, 1 mM β-glycerol phosphate, 1 mM sodium azide, 1 mM sodium orthovanadate). The samples were immediately spun down in a refrigerated centrifuge and the pellets were lysed with phospho-stop buffer plus 1% Triton X-100, before separating on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferring to polyvinylidene difluoride and immunoblotting.
Immunoprecipitation and immunoblotting
NK-92 cells (40 million/sample) were stimulated with pervanadate, lysed, and sequentially immunoprecipitated with mAbs that were precoupled to protein G-agarose (Upstate Biotechnology, Charlottesville, VA), as described.27 Immunoprecipitates were separated by SDS-PAGE, transferred to polyvinylidene difluoride, and sequentially immunoblotted using horseradish peroxidase (HRP)-conjugated antiphosphotyrosine (Upstate Biotechnology), rabbit anti-BCAP polyclonal Ab22 + HRP-protein G (Calibochem, San Diego, CA), and then anti-p85 subunit of PI3-K (Santa Cruz Biotechnology, Santa Cruz, CA) plus HRP-protein G. The membrane was stripped between each antibody blot as previously described.27 Whole cell lysates were prepared, lysed in SDS-PAGE sample buffer, and probe sonicated to shear DNA.
BrdU uptake assays
Nine WT and 9 BCAP−/− mice were fed drinking water containing 0.8 mg/mL bromodeoxyaridine (BrdU; Sigma-Aldrich) plus 1 mg/mL dextrose for 20 days before the initial time point. After 0, 14, and 65 days, splenocytes from 3 WT and 3 BCAP−/− mice were harvested and stained with anti-NK1.1 allophycocyanin [APC], anti-CD3ϵ (Pacific Blue), and anti-Ly49I (PE; eBioscience). Cells were fixed, permeabilized, incubated for 30 minutes with DNase solution (150 mM NaCl, 4.2 mM MgCl2, 225 μg/mL DNase I (Roche), pH 5), and stained with fluorescein isothiocyanate (FITC)–conjugated anti-BrdU (BD Biosciences PharMingen).
Apoptosis
Freshly harvested splenocytes were cultured in complete RPMI 1640 at 37°C with 7% CO2 after erythrocyte lysis. Apoptosis was induced by either the absence of IL-2 overnight, or the addition 5 μM staurosporine for 3 hours. Control cultures received 1000 U/mL recombinant human IL-2 in the overnight assay. Some samples were cultured for 18 hours with IL-2 and then 24 hours without IL-2. Cells were then stained with anti-NK1.1 (APC), anti-CD3ϵ (Pacific Blue), and annexin V (FITC; BD Biosciences). Some experiments also included antibodies to Ly49I (PE) and CD11b (Alexa Fluor 750 APC).
Results
BCAP expression in NK cells
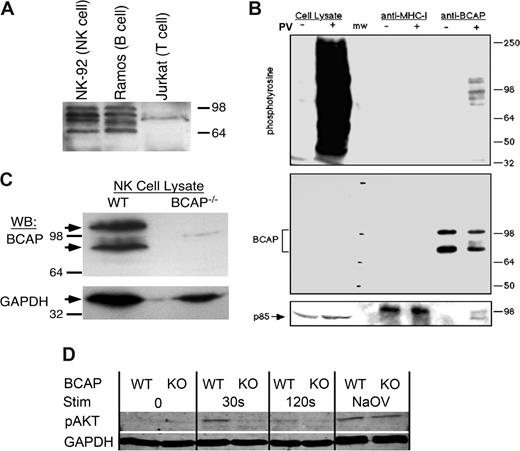
Previous studies detected BCAP in B cells and myeloid cells, but not T cells.22 We detected strong expression of BCAP in the NK-92 NK-like cell line and the Ramos B-cell line, but no expression in Jurkat T cells (Figure 1A). Multiple BCAP bands were observed between 70 and 100 kDa, which were previously shown to be alternative mRNA splice products.22 BCAP was also detected in the NK3.3 and KHYG-1 human NK-like cell lines (data not shown). We further demonstrated an association of the p85 subunit of PI3-K with tyrosine-phosphorylated BCAP from pervanadate-stimulated NK-92 cells (Figure 1B). Primary mouse NK cells were also found to express BCAP, but NK cells from BCAP−/− mice23 lacked the protein (Figure 1C). Thus, BCAP is strongly expressed in both murine and human NK cells, can recruit PI3-K, and is not expressed by NK cells from the BCAP−/− mouse.
BCAP is expressed in human and murine NK cells, recruits PI3-K upon tyrosine phosphorylation, and is involved in Akt signaling. (A) Whole cell lysates from (0.5 million cells/lane) were separated by SDS-PAGE and immunoblotted for BCAP. Multiple bands are the result of alternative splicing,22 and the weak band near 85 kDa was nonspecific in all lysates tested. (B) NK-92 cells (40 million/sample) were treated with (+) or without (−) pervanadate for 10 minutes at 37°C, lysed, immunoprecipitated with anti-MHC-I mAb (W6/32) and anti-BCAP mAb (4L8E6), separated by SDS-PAGE, and immunoblotted for phosphotyrosine, BCAP, and the p85 subunit of PI3-K. (C) NK cells from wild-type (WT) and BCAP−/− mice were MACS purified and cultured with IL-2 for 1 week. Whole cell lysates (106/sample) were separated by SDS-PAGE and immunoblotted for BCAP and GAPDH. (D) Purified NK cells from WT or BCAP−/− mice were sorted and cultured in IL-2 for 8 days, then stimulated with biotinylated anti-NK1.1 antibody and streptavidin. Cells were lysed at the indicated times after streptavidin addition, and Akt phosphorylation was resolved by Western blot. Immunoblotting for GAPDH levels was performed as a loading control. No streptavidin was added to the time 0 samples, and the positive control was treated with pervanadate for 10 minutes. The result is representative of 4 separate experiments.
BCAP is expressed in human and murine NK cells, recruits PI3-K upon tyrosine phosphorylation, and is involved in Akt signaling. (A) Whole cell lysates from (0.5 million cells/lane) were separated by SDS-PAGE and immunoblotted for BCAP. Multiple bands are the result of alternative splicing,22 and the weak band near 85 kDa was nonspecific in all lysates tested. (B) NK-92 cells (40 million/sample) were treated with (+) or without (−) pervanadate for 10 minutes at 37°C, lysed, immunoprecipitated with anti-MHC-I mAb (W6/32) and anti-BCAP mAb (4L8E6), separated by SDS-PAGE, and immunoblotted for phosphotyrosine, BCAP, and the p85 subunit of PI3-K. (C) NK cells from wild-type (WT) and BCAP−/− mice were MACS purified and cultured with IL-2 for 1 week. Whole cell lysates (106/sample) were separated by SDS-PAGE and immunoblotted for BCAP and GAPDH. (D) Purified NK cells from WT or BCAP−/− mice were sorted and cultured in IL-2 for 8 days, then stimulated with biotinylated anti-NK1.1 antibody and streptavidin. Cells were lysed at the indicated times after streptavidin addition, and Akt phosphorylation was resolved by Western blot. Immunoblotting for GAPDH levels was performed as a loading control. No streptavidin was added to the time 0 samples, and the positive control was treated with pervanadate for 10 minutes. The result is representative of 4 separate experiments.
Defect in Akt signaling in BCAP−/− NK cells
Phosphorylation of Akt was profoundly suppressed in IL-2–cultured BCAP−/− NK cells stimulated through NK1.1 crosslinking (Figure 1D). NK1.1 is an immunoreceptor tyrosine-based activation motif (ITAM)–mediated signaling receptor that would have capacity to stimulate PI3-K in NK cells,28 and Akt requires PI3-K activity to generate a membrane-proximal binding site for its pleckstrin homology domain.29 The severe reduction in Akt phosphorylation provides evidence that BCAP acts as an adaptor for PI3K in NK cells and supports the hypothesis that BCAP acts as a positive regulator of activation signaling in both NK cells and B cells.22,23,30
Lymphocyte populations in BCAP−/− mice
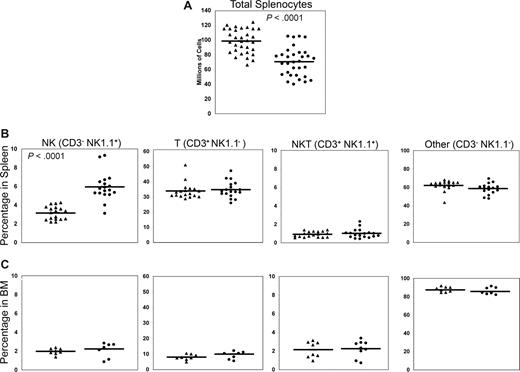
We next analyzed the phenotype and function of NK cells from BCAP−/− mice, compared with those from normal WT C57BL/6 mice. The first striking observations in BCAP−/− mice were a reduction in the total number of splenic lymphocytes and an increased percentage of splenic NK cells (Figure 2). The mean number of splenocytes harvested from WT mice was 98 plus or minus 16 million, whereas 70 plus or minus 19 million cells were harvested from BCAP−/− mice. The percentage of NK cells was nearly doubled in the spleens of BCAP−/− mice (5.9% ± 1.5% NK cells compared with 3.2% ± 0.7% in the WT). Taken together, these data indicate a 32% increase in the absolute number of splenic NK cells in BCAP−/− mice (WT: 3.2 ± 1.1 × 106; knockout [KO]: 4.4 ± 1.3 × 106, P < .05, n = 10). Percentages of splenic T, B, and NKT cells were not significantly altered, consistent with previous results.23 The percentage of NK1.1+CD3− NK cells was unchanged in the bone marrow (Figure 2), suggesting that the increase in splenic NK cells arose in late maturation.
Decreased total lymphocytes and increased fraction of NK cells in spleens from BCAP−/− mice. Each point represents an individual wild-type (WT, ▴) or BCAP−/− (●) mouse 7 to 24 weeks of age, and mean values are represented by horizontal lines. (A) The total number of splenocytes harvested was counted manually, and dead cells were excluded by trypan blue staining. P value was calculated using a 2-tailed Student t test. (B) Percentages of cells in spleens that are CD3−NK1.1+ (NK), CD3+NK1.1− (T), CD3+NK1.1+ (NKT), or CD3−NK1.1− (Other). “Other” cells are almost exclusively B cells (data not shown). P values are as follows: NK < .001, T = .61, NKT = .51, and Other = .07. (C) Lymphocyte populations in the bone marrow of WT and BCAP−/− mice. No statistically significant difference was found in the percentages of bone marrow lymphocyte subpopulations.
Decreased total lymphocytes and increased fraction of NK cells in spleens from BCAP−/− mice. Each point represents an individual wild-type (WT, ▴) or BCAP−/− (●) mouse 7 to 24 weeks of age, and mean values are represented by horizontal lines. (A) The total number of splenocytes harvested was counted manually, and dead cells were excluded by trypan blue staining. P value was calculated using a 2-tailed Student t test. (B) Percentages of cells in spleens that are CD3−NK1.1+ (NK), CD3+NK1.1− (T), CD3+NK1.1+ (NKT), or CD3−NK1.1− (Other). “Other” cells are almost exclusively B cells (data not shown). P values are as follows: NK < .001, T = .61, NKT = .51, and Other = .07. (C) Lymphocyte populations in the bone marrow of WT and BCAP−/− mice. No statistically significant difference was found in the percentages of bone marrow lymphocyte subpopulations.
Analysis of NK cell development
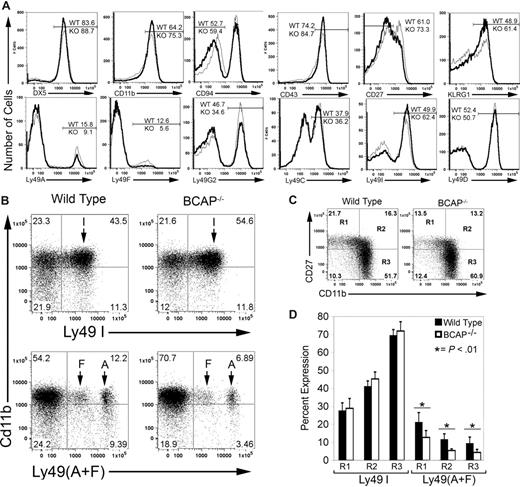
We observed a greater frequency of mature NK cells in BCAP−/− mice, as defined by surface phenotypes of DX5high, CD11bhigh, CD94low, CD43high, CD27low, and KLRG1high (Figure 3A; Table 1). These receptor expression patterns are characteristic of mature NK cells.31-34 No measurable difference was found in the expression of NK1.1, NKG2D, 2B4, CD16, or CD62L (data not shown). Thus, a larger proportion of the NK cells in BCAP−/− mice are mature, but activating receptor expression is similar to that of WT mice.
Increased mature splenic NK cells and shift in expression of Ly49 receptors in BCAP−/− mice. (A) Surface staining of NK cells (CD3−NK1.1+) is shown, with BCAP−/− cells represented by the thicker line. Histograms with equal numbers of cells are compared, and the percentage of cells in each gate is indicated. (B) Expression of inhibitory Ly49 receptors as a function of CD11b expression. NK cells were stained for CD11b and either Ly49A and Ly49F or Ly49I. (C) Relationship between CD11b expression and CD27 expression in BCAP−/− and wild-type NK cells. Quadrants labeled R1, R2, and R3,31 define immature NK cells (R1) and 2 functionally distinct mature subsets (R2 and R3), and the percentage of cells in each quadrant is listed. (D) Expression of self-recognizing and non–self-recognizing inhibitory Ly49 receptors on NK cells from wild-type (■) or BCAP−/− mice (□) as a function of maturity. Cells within R1, R2, and R3, as in panel C, were further stained for either Ly49I, or Ly49A and Ly49F. Error bars show the SD from 5 mice (*difference with P < .01). All data shown in this figure are representative of at least 3 experiments.
Increased mature splenic NK cells and shift in expression of Ly49 receptors in BCAP−/− mice. (A) Surface staining of NK cells (CD3−NK1.1+) is shown, with BCAP−/− cells represented by the thicker line. Histograms with equal numbers of cells are compared, and the percentage of cells in each gate is indicated. (B) Expression of inhibitory Ly49 receptors as a function of CD11b expression. NK cells were stained for CD11b and either Ly49A and Ly49F or Ly49I. (C) Relationship between CD11b expression and CD27 expression in BCAP−/− and wild-type NK cells. Quadrants labeled R1, R2, and R3,31 define immature NK cells (R1) and 2 functionally distinct mature subsets (R2 and R3), and the percentage of cells in each quadrant is listed. (D) Expression of self-recognizing and non–self-recognizing inhibitory Ly49 receptors on NK cells from wild-type (■) or BCAP−/− mice (□) as a function of maturity. Cells within R1, R2, and R3, as in panel C, were further stained for either Ly49I, or Ly49A and Ly49F. Error bars show the SD from 5 mice (*difference with P < .01). All data shown in this figure are representative of at least 3 experiments.
Increased frequency of self-recognizing mature splenic NK cells from BCAP−/− mice in a C57BL/6 background
| Receptor . | Wild-type, % . | BCAP−/−, % . |
|---|---|---|
| DX5high | 86.5 ± 3.9 | 90.9 ± 2.9* |
| CD11bhigh | 66.7 ± 5.6 | 76.5 ± 4.1* |
| CD94low | 53.0 ± 2.5 | 58.7 ± 2.5* |
| CD43high | 74.6 ± 4.3 | 84.6 ± 3.6* |
| CD27low | 62.7 ± 5.4 | 74.1 ± 5.7* |
| KLRG1+ | 46.7 ± 4.2 | 56.5 ± 7.2* |
| Ly49A+ | 14.9 ± 2.9 | 11.1 ± 3.1* |
| Ly49F+ | 11.1 ± 1.6 | 5.3 ± 0.6* |
| Ly49G2+ | 47.5 ± 1.9 | 36.9 ± 5.7* |
| Ly49C+ | 37.0 ± 6.2 | 37.2 ± 6.2 |
| Ly49I+ | 51.1 ± 3.8 | 63.2 ± 3.9* |
| Ly49D+ | 50.3 ± 2.5 | 47.2 ± 3.7 |
| Receptor . | Wild-type, % . | BCAP−/−, % . |
|---|---|---|
| DX5high | 86.5 ± 3.9 | 90.9 ± 2.9* |
| CD11bhigh | 66.7 ± 5.6 | 76.5 ± 4.1* |
| CD94low | 53.0 ± 2.5 | 58.7 ± 2.5* |
| CD43high | 74.6 ± 4.3 | 84.6 ± 3.6* |
| CD27low | 62.7 ± 5.4 | 74.1 ± 5.7* |
| KLRG1+ | 46.7 ± 4.2 | 56.5 ± 7.2* |
| Ly49A+ | 14.9 ± 2.9 | 11.1 ± 3.1* |
| Ly49F+ | 11.1 ± 1.6 | 5.3 ± 0.6* |
| Ly49G2+ | 47.5 ± 1.9 | 36.9 ± 5.7* |
| Ly49C+ | 37.0 ± 6.2 | 37.2 ± 6.2 |
| Ly49I+ | 51.1 ± 3.8 | 63.2 ± 3.9* |
| Ly49D+ | 50.3 ± 2.5 | 47.2 ± 3.7 |
Values are means plus or minus SD from at least 7 mice.
Wild-type versus BCAP−/− comparison; P values are < .05 as calculated by a two-tailed Student t test.
A greater percentage of NK cells from BCAP−/− mice were also found to express Ly49I, which recognize H-2b (the MHC-I haplotype of C57BL/6 mice), whereas fewer cells express Ly49A, G2, and F, which recognize other MHC-I haplotypes that are absent in these mice (Figure 3A; Table 1). This represents a shift trending toward more self-recognizing cells, but the frequency of cells expressing the H2b-recognizing inhibitory Ly49C receptor was unchanged. In addition, the frequency of NK cells expressing the activating receptor Ly49D was unaffected by the loss of BCAP. Furthermore, the intensity of Ly49 surface staining was not altered in BCAP−/− mice, indicating that surface expression levels are unchanged. All values shown are representative of at least 7 mice 7 to 24 weeks of age, but experiments on mice up to 39 weeks of age yielded similar results (not shown).
BCAP−/− mice also have an increased frequency of mature NK cells (CD11bhigh) that express the SRIR Ly49I and substantial declines in cells expressing Ly49 that do not recognize self MHC-I (Ly49A or Ly49F) or cells that are immature (CD11blow) and lack Ly49I expression (Figure 3B). Hayakawa and Smyth recently demonstrated that peripheral NK cells could be divided into 3 functionally distinct subsets according to expression of CD11b and CD27 (R1, R2, and R3).31,35 R1 cells are immature, whereas R2 and R3 fractionate the mature cells into 2 functionally distinct populations. We found a substantially greater accumulation of NK cells in the CD11bhighCD27low (R3) stage in BCAP−/− mice (Figure 3C). As previously reported,31 the percentage of NK cells expressing Ly49I increases steadily as cells progress from R1 to R3 in WT mice (Figure 3D). Our analysis further revealed that the percentage of cells expressing Ly49A or Ly49F declines substantially in R2 and R3 fractions, supporting the hypothesis that self-recognition is an important factor in selecting which NK cells reach a fully mature state. NK cells from BCAP−/− mice showed only modest increases in percentages of self-recognizing cells but significant declines in fractions of Ly49A- and Ly49F-expressing cells in all 3 stages (Figure 3D). Therefore, the skewing toward self-recognizing NK cells in BCAP−/− mice is the result of the increased accumulation of R3 cells that characteristically express a greater percentage of self-recognizing Ly49.
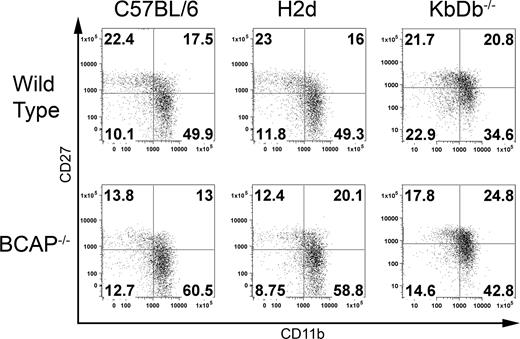
In light of the apparent shifts toward expression of SRIRs, we tested whether this is driven by MHC-I ligand by crossing the BCAP−/− mice to both MHC-I–deficient (KbDb−/−) and Ly49C/I/A/G2-recognizing H2d congenic backgrounds. The KbDb−/− mouse does not express classic MHC-I but does express Qa-1, which is the ligand for the CD94/NKG2A SRIR.36 As shown in Tables 1 and S1, the Ly49 receptor expression profile in H2d congenic mice is essentially the same as BCAP−/− mice in the H2b C57BL/6 background, which demonstrates that the altered frequency of Ly49 receptor expression in BCAP−/− mice is not driven by self-ligand. Similar shifts in the Ly49 repertoire were also observed in BCAP−/− mice in the KbDb−/− background (Table 2), further emphasizing that the altered expression pattern of Ly49 receptors in BCAP−/− mice is independent of self-ligand. Interestingly, in the BCAP+/+ MHC-I–deficient background, far fewer cells reach the terminal stage of differentiation (CD27low, CD11bhigh, KLRG-1+) compared with the WT C57BL/6 and H2d congenic backgrounds (Tables 1,2, and S1; Figure 4). Elimination of BCAP, however, still drove more MHC-I–deficient NK cells to become fully mature, thus demonstrating that the enhanced maturation is also independent of MHC-I.
Cell surface receptors on splenic NK cells from KbDb−/− mice with and without BCAP
| Receptor . | Wild-type, % . | BCAP−/−, % . |
|---|---|---|
| DX5high | 83.9 ± 8.2 | 90.8 ± 3.9 |
| CD11bhigh | 62.3 ± 10.0 | 69.7 ± 9.2 |
| CD94low | 53.2 ± 5.0 | 51.2 ± 3.0 |
| CD43high | 76.0 ± 8.3 | 82.9 ± 5.2 |
| CD27low | 47.2 ± 8.7 | 48.5 ± 8.0 |
| KLRG1+ | 21.5 ± 3.4 | 31.8 ± 3.2 |
| Ly49A+ | 15.8 ± 1.4 | 8.6 ± 1.6 |
| Ly49F+ | 12.5 ± 2.4 | 6.1 ± 1.5 |
| Ly49G2+ | 48.7 ± 3.7 | 35.4 ± 2.4 |
| NKG2A+ | 40.4 ± 2.4 | 41.6 ± 3.4 |
| Ly49I+ | 57.6 ± 3.5 | 63.1 ± 3.7 |
| Ly49D+ | 43.6 ± 3.3 | 45.4 ± 4.3 |
| Receptor . | Wild-type, % . | BCAP−/−, % . |
|---|---|---|
| DX5high | 83.9 ± 8.2 | 90.8 ± 3.9 |
| CD11bhigh | 62.3 ± 10.0 | 69.7 ± 9.2 |
| CD94low | 53.2 ± 5.0 | 51.2 ± 3.0 |
| CD43high | 76.0 ± 8.3 | 82.9 ± 5.2 |
| CD27low | 47.2 ± 8.7 | 48.5 ± 8.0 |
| KLRG1+ | 21.5 ± 3.4 | 31.8 ± 3.2 |
| Ly49A+ | 15.8 ± 1.4 | 8.6 ± 1.6 |
| Ly49F+ | 12.5 ± 2.4 | 6.1 ± 1.5 |
| Ly49G2+ | 48.7 ± 3.7 | 35.4 ± 2.4 |
| NKG2A+ | 40.4 ± 2.4 | 41.6 ± 3.4 |
| Ly49I+ | 57.6 ± 3.5 | 63.1 ± 3.7 |
| Ly49D+ | 43.6 ± 3.3 | 45.4 ± 4.3 |
Values are means plus or minus SD from at least 3 mice.
Effects of self-ligand on maturation. Fresh splenocytes were stained with anti-CD3ϵ, anti-NK1.1, anti-CD27, and anti-CD11b. Propidium iodide was added to exclude dead cells. NK cells are gated as NK1.1+CD3− and divided into subpopulations according to the expression levels of CD27 and CD11b as in Figure 3C. Surface phenotype of wild-type and BCAP−/− NK cells from C57BL/6, H2d congenic, and KbDb−/− backgrounds are shown. All of the data are representative of at least 3 mice. Numbers on plots are the percentages of total NK cells in each quadrant.
Effects of self-ligand on maturation. Fresh splenocytes were stained with anti-CD3ϵ, anti-NK1.1, anti-CD27, and anti-CD11b. Propidium iodide was added to exclude dead cells. NK cells are gated as NK1.1+CD3− and divided into subpopulations according to the expression levels of CD27 and CD11b as in Figure 3C. Surface phenotype of wild-type and BCAP−/− NK cells from C57BL/6, H2d congenic, and KbDb−/− backgrounds are shown. All of the data are representative of at least 3 mice. Numbers on plots are the percentages of total NK cells in each quadrant.
Apoptosis of NK cells from BCAP−/− mice
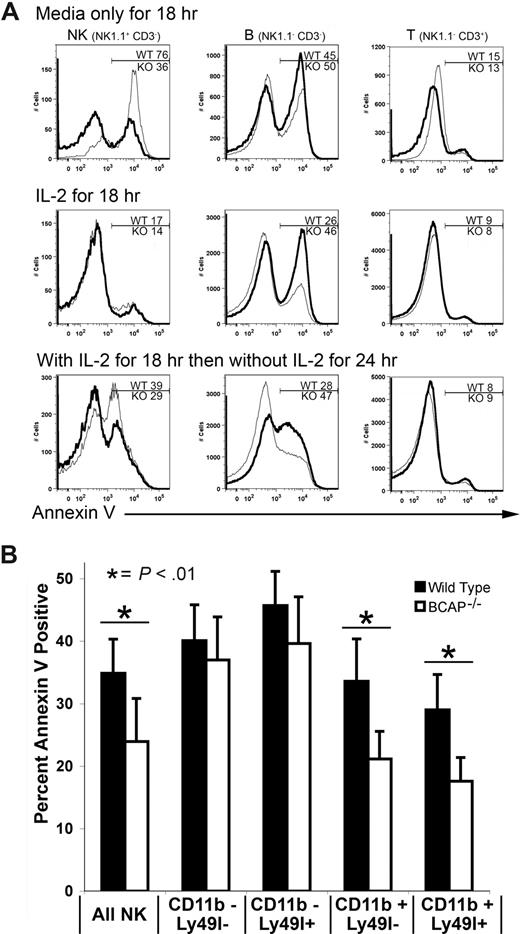
We speculated that the increased frequency of long-lived, self-recognizing NK cells might result from enhanced survival because of reduced sensitivity to apoptosis. To test this, we determined annexin V staining on NK cells after culture without IL-2. Surprisingly, whereas cultured BCAP−/− B cells are more susceptible to apoptosis, as previously shown,23 BCAP−/− NK cells are more resistant to apoptosis than their WT counterparts (Figure 5A). In contrast, T cells, which do not express BCAP, showed no difference in susceptibility to apoptosis. Similar results were obtained on inducing apoptosis with etoposide (data not shown). These data suggest that a greater resistance to apoptosis may contribute to the accumulation of NK cells in BCAP−/− mice.
Reduced apoptosis by mature splenic NK cells from BCAP−/− mice. (A) Fresh splenic lymphocytes were incubated overnight at 37°C in either medium alone or medium containing IL-2. After incubating overnight (18 hours), the cells were stained with anti-NK1.1, anti-CD3ϵ, and annexin V, then analyzed by flow cytometry. Results for BCAP−/− samples are represented by a thicker line. Percentages of apoptotic BCAP−/− (KO) and wild-type (WT) cells in the gated regions are shown. Top panels are cells incubated overnight in medium alone, middle panels show cells incubated overnight in medium with IL-2, and cells incubated overnight with IL-2, then 24 hours without IL-2 are shown in the bottom panel. (B) Fresh splenic lymphocytes were incubated in the absence of IL-2 for 3 hours at 37°C with or without 5 μM staurosporine, then stained with anti-NK1.1, anti-CD3ϵ, anti-CD11b, annexin V, and anti-Ly49I. Values are means plus or minus SD of 6 WT and 6 BCAP−/− mice (*difference with P < .01).
Reduced apoptosis by mature splenic NK cells from BCAP−/− mice. (A) Fresh splenic lymphocytes were incubated overnight at 37°C in either medium alone or medium containing IL-2. After incubating overnight (18 hours), the cells were stained with anti-NK1.1, anti-CD3ϵ, and annexin V, then analyzed by flow cytometry. Results for BCAP−/− samples are represented by a thicker line. Percentages of apoptotic BCAP−/− (KO) and wild-type (WT) cells in the gated regions are shown. Top panels are cells incubated overnight in medium alone, middle panels show cells incubated overnight in medium with IL-2, and cells incubated overnight with IL-2, then 24 hours without IL-2 are shown in the bottom panel. (B) Fresh splenic lymphocytes were incubated in the absence of IL-2 for 3 hours at 37°C with or without 5 μM staurosporine, then stained with anti-NK1.1, anti-CD3ϵ, anti-CD11b, annexin V, and anti-Ly49I. Values are means plus or minus SD of 6 WT and 6 BCAP−/− mice (*difference with P < .01).
We next tested if there was a correlation between the increased percentage of mature and self-recognizing NK cells in BCAP−/− mice and the decreased susceptibility of these cells to apoptosis. We treated cells with staurosporine because it initiates apoptosis independent of cell proliferation. As shown in Figure 5B, mature WT NK cells are somewhat more resistant to apoptosis, but this resistance is significantly magnified in mature NK cells from BCAP−/− mice. Taken together, NK cells from BCAP−/− mice are more resistant to apoptosis, and the resistance is most pronounced in the mature subpopulations.
Persistence of self-recognizing NK cells in BCAP−/− mice
To examine the accumulation and survival of self-recognizing NK cells in vivo, we loaded BCAP−/− and WT mice with BrdU for 20 days and then observed the persistence of NK cells possessing SRIRs over time. Whereas nearly equivalent percentages of cells were initially labeled, a greater percentage of NK cells persisted in BCAP−/− mice after 65 days (Table 3). When the BrdU+ NK cells in these mice were analyzed for expression of Ly49I, the WT mice showed a slightly greater persistence of self-recognizing NK cells over time. Alternatively, BCAP−/− mice started with a lower percentage of self-recognizing NK cells within the BrdU+ pool, but this percentage increased substantially to surpass the percentage in WT mice by day 65. The cumulative results indicate a survival advantage by self-recognizing NK cells and suggest that the greater number of NK cells present in BCAP−/− mice derive from longer persistence of such cells.
Evolution of the Ly49I repertoire in NK cells over time
| Days after pulsing with BrdU* . | % BrdU+ NK cells in WT mice† . | % BrdU+ NK cells in BCAP−/− mice† . | % Ly49I+ NK cells within BrdU+ cells of WT mice† . | % Ly49I+ NK cells within BrdU+ cells of BCAP−/− mice† . |
|---|---|---|---|---|
| Day 0 | 38.4 ± 12.6 | 38.7 ± 8.0 | 38.3 ± 2.6 | 31.8 ± 4.8 |
| Day 14 | 32.6 ± 6.7 | 36.8 ± 2.0 | 39.5 ± 5.0 | 38.7 ± 2.8 |
| Day 65 | 6.2 ± 1.9 | 8.9 ± 1.0 | 44.1 ± 9.3 | 54.7 ± 2.5 |
| Days after pulsing with BrdU* . | % BrdU+ NK cells in WT mice† . | % BrdU+ NK cells in BCAP−/− mice† . | % Ly49I+ NK cells within BrdU+ cells of WT mice† . | % Ly49I+ NK cells within BrdU+ cells of BCAP−/− mice† . |
|---|---|---|---|---|
| Day 0 | 38.4 ± 12.6 | 38.7 ± 8.0 | 38.3 ± 2.6 | 31.8 ± 4.8 |
| Day 14 | 32.6 ± 6.7 | 36.8 ± 2.0 | 39.5 ± 5.0 | 38.7 ± 2.8 |
| Day 65 | 6.2 ± 1.9 | 8.9 ± 1.0 | 44.1 ± 9.3 | 54.7 ± 2.5 |
Each data point represents the mean plus or minus SD from 3 mice.
Wild-type and BCAP−/− mice were pulsed with 5′-BrdU in drinking water for 20 days before day 0. Splenocytes were harvested on the indicated days after pulsing for analysis by flow cytometry.
NK cells were gated as NK1.1+CD3−. The percentage of self-recognizing NK cells was identified by costaining with anti-Ly49I.
Immunologic function of BCAP−/− NK cells
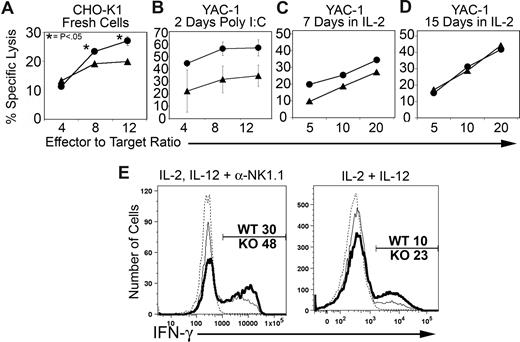
To test for functional impacts resulting from BCAP deficiency, we measured the ability of WT and BCAP−/− NK cells to lyse target cells, produce IFN-γ, and clear virus. We found that NK cells from BCAP−/− mice elicited a moderate increase in cytotoxicity toward multiple target cells. Increased cytolytic activity was observed against CHO-K1 (Figure 6A), Yac-1 (Figures 6B-D), and L929 (not shown). Increased in vitro cytotoxicity was evident in freshly isolated NK cells from unstimulated mice (Figure 6A) or mice that were pretreated with poly I:C (Figure 6B), as well as NK cells cultured for a week or less in IL-2 (Figure 6C). Importantly, activating receptors known to be involved in killing these targets were expressed at identical levels and frequencies on BCAP−/− and WT NK cells (NKG2D for YAC-1, Ly49D for CHO-K1, and NK1.1 for L929). Long-term culturing in the presence of IL-2 eliminated the difference in cytotoxicity against YAC-1 (Figure 6D). We also measured in vitro NK-cell IFN-γ responses. A significantly higher percentage of BCAP−/− NK cells produced IFN-γ when stimulated with a mixture of IL-2, IL-12, and plate-bound anti-NK1.1, or only IL-2 and IL-12, as shown in Figure 6E.
BCAP−/− NK cells exhibit enhanced immune functions. (A) Cytotoxicity response by freshly harvested wild-type (▴) and BCAP−/− (●) NK cells against CHO-K1 targets. Splenocytes were apportioned into wells along with target cells to achieve the stated effector (CD3−, NK1.1+) to target ratio. The data shown represent the means plus or minus SE of 3 separate experiments. In some cases, the error bars are smaller than the data point icon. P values were calculated by a 2-tailed Student t test. (B) Cytotoxicity against YAC-1 targets by freshly isolated NK cells from mice prestimulated with poly I:C. The data shown represent the means plus or minus SE of 2 separate experiments. (C,D) Cytotoxicity against YAC-1 by sorted and IL-2 cultured NK cells (1000 U/mL) for 7 days (C) or 15 days (D) The data shown represent a single experiment but are representative of at least 3 experiments that were performed under similar conditions. (E) IFN-γ response by stimulated NK cells. Splenic lymphocytes were cultured for 5 hours with or without combinations of 500 U/mL IL-2, 10 ng/mL IL-12, and plate-bound anti-NK1.1. Brefeldin A (10 μg/mL) was added after the first hour. Stimulated BCAP−/− NK cells are shown as a thick line, stimulated wild-type cells as a thin line, and the unstimulated wild-type control as a thin dashed line (< 1% IFN-γ+). Percentages of IFN-γ+ NK cells are indicated. Data are representative of at least 3 experiments.
BCAP−/− NK cells exhibit enhanced immune functions. (A) Cytotoxicity response by freshly harvested wild-type (▴) and BCAP−/− (●) NK cells against CHO-K1 targets. Splenocytes were apportioned into wells along with target cells to achieve the stated effector (CD3−, NK1.1+) to target ratio. The data shown represent the means plus or minus SE of 3 separate experiments. In some cases, the error bars are smaller than the data point icon. P values were calculated by a 2-tailed Student t test. (B) Cytotoxicity against YAC-1 targets by freshly isolated NK cells from mice prestimulated with poly I:C. The data shown represent the means plus or minus SE of 2 separate experiments. (C,D) Cytotoxicity against YAC-1 by sorted and IL-2 cultured NK cells (1000 U/mL) for 7 days (C) or 15 days (D) The data shown represent a single experiment but are representative of at least 3 experiments that were performed under similar conditions. (E) IFN-γ response by stimulated NK cells. Splenic lymphocytes were cultured for 5 hours with or without combinations of 500 U/mL IL-2, 10 ng/mL IL-12, and plate-bound anti-NK1.1. Brefeldin A (10 μg/mL) was added after the first hour. Stimulated BCAP−/− NK cells are shown as a thick line, stimulated wild-type cells as a thin line, and the unstimulated wild-type control as a thin dashed line (< 1% IFN-γ+). Percentages of IFN-γ+ NK cells are indicated. Data are representative of at least 3 experiments.
To determine the in vivo significance of this enhanced NK- cell function in BCAP−/− mice, we tested for resistance to infection by ectromelia virus during the first week after infection, when NK-cell activity is important.37 NK cells have previously been shown to play a critical role in clearance of ectromelia virus.38,39 Footpad injections of ectromelia virus were administered to mice and the splenic viral titers were measured 5 or 7 days later. BCAP−/− mice demonstrated a trend toward early enhanced clearance of the virus in 3 experiments (Figure S2). The enhanced early viral clearance most probably derives from a combined contribution of the increased number of peripheral NK cells and enhanced activity mediated by a greater percentage of mature cells.
Functional effects of maturity and self-recognition
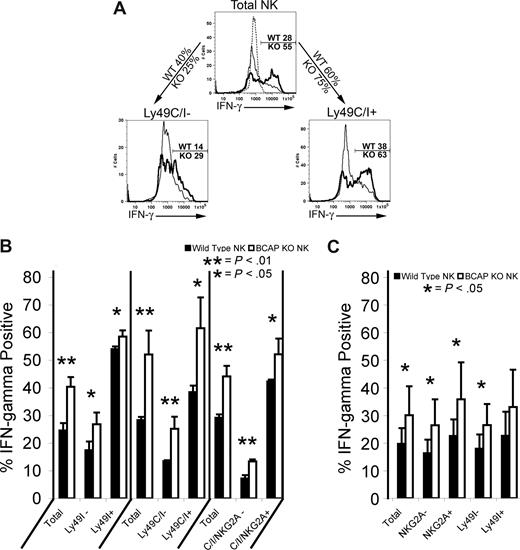
Given the augmented NK-cell maturation and function, we investigated IFN-γ responses within NK cell subsets stratified by expression of SRIRs Ly49C, Ly49I, and NKG2A. Consistent with previous studies,13,15 we show that the subset of freshly isolated murine NK cells expressing SRIRs generates stronger IFN-γ responses than cells lacking these receptors,13,15 and the difference was enhanced if all 3 SRIRs were used to distinguish self-recognition (Figure 7A,B; in the first set of 6 bars in Figure 7B, a combination of YLI-90 and 5E6 was used, but the bars are labeled “Ly49I” in view of our results in Figure S1). NK cells from the BCAP−/− mouse were significantly better at producing IFN-γ in every subset analyzed (Figure 7B). The advantage in IFN-γ production afforded to MHC-I–sufficient cells expressing Ly49I or NKG2A was not evident in the MHC-I–deficient KbDb−/− mice, but, importantly, the advantage of NK cells from the BCAP−/− mice remained in all subsets, further demonstrating that the enhanced function of BCAP−/− NK cells is independent of Ly49 ligand expression (Figure 7C).
IFN-γ production by NK cells from the spleens of WT and BCAP−/− mice as a function of SRIR expression. (A) Fresh splenocytes from C57BL/6 background mice were incubated alone or with plate-bound anti-NK1.1, 500 U/mL IL-2, and 10 ng/mL IL-12; 10 μg/mL brefeldin A was added 1 hour later, and the cells were cultured 4 more hours. Cells were surface stained, then fixed, permeabilized, and stained for intracellular IFN-γ. The thicker line represents BCAP−/− mice, and the dashed line represents unstimulated control. Percentages of IFN-γ+ cells in WT and BCAP−/− NK cells are listed on gates. Percentages on arrows are the percentage of NK cells on each mouse that are either negative for both Ly49C and Ly49I (left arrow) or express at least one of these SRIRs (right arrow). (B) Mean IFN-γ responses in NK-cell subsets from 3 C57BL/6 background mice analyzed, as described in panel A. The mean percentages plus or minus SD of IFN-γ+ wild-type (■) and BCAP−/− NK cells (□) are shown. P values were calculated by applying a 2-tailed Student t test (*, **). (C) Mean IFN-γ responses of 7 pairs of MHC-I–deficient mice as described in panel A. The mean percentages plus or minus SD of IFN-γ+ wild-type (■) and BCAP−/− NK cells (□) within indicated subsets (NKG2A of Ly49I expression) are shown (*).
IFN-γ production by NK cells from the spleens of WT and BCAP−/− mice as a function of SRIR expression. (A) Fresh splenocytes from C57BL/6 background mice were incubated alone or with plate-bound anti-NK1.1, 500 U/mL IL-2, and 10 ng/mL IL-12; 10 μg/mL brefeldin A was added 1 hour later, and the cells were cultured 4 more hours. Cells were surface stained, then fixed, permeabilized, and stained for intracellular IFN-γ. The thicker line represents BCAP−/− mice, and the dashed line represents unstimulated control. Percentages of IFN-γ+ cells in WT and BCAP−/− NK cells are listed on gates. Percentages on arrows are the percentage of NK cells on each mouse that are either negative for both Ly49C and Ly49I (left arrow) or express at least one of these SRIRs (right arrow). (B) Mean IFN-γ responses in NK-cell subsets from 3 C57BL/6 background mice analyzed, as described in panel A. The mean percentages plus or minus SD of IFN-γ+ wild-type (■) and BCAP−/− NK cells (□) are shown. P values were calculated by applying a 2-tailed Student t test (*, **). (C) Mean IFN-γ responses of 7 pairs of MHC-I–deficient mice as described in panel A. The mean percentages plus or minus SD of IFN-γ+ wild-type (■) and BCAP−/− NK cells (□) within indicated subsets (NKG2A of Ly49I expression) are shown (*).
Discussion
Our studies have clarified several aspects of the intertwined roles between NK-cell self-recognition, maturation, and functional competence, which supports the work of others.6,13,15,31 In the C57BL/6 background, we observed a significant decline in the fraction of NK cells expressing Ly49 that are not SRIRs (either Ly49A or Ly49F) as the cells proceed from the immature (R1) to mature (R2) stage, along with a commensurate increase in terminally differentiated NK cells that express SRIRs. These observations strongly suggest that expression of a SRIR promotes the transition from immature to mature effector stages. NK cells from the BCAP−/− mice exhibit further declines in the expression of Ly49A and Ly49F, and the effect is independent of MHC-I ligands.
Numerous additional aspects of the maturation process are magnified in NK cells from the BCAP−/− mouse, including (1) increased percentage of terminally differentiated NK cells, (2) a larger advantage in resisting apoptosis within the mature NK cell pool, (3) increased long-term accumulation of mature self-recognizing NK cells in BrdU pulse-chase experiments, and (4) potentiated IFN-γ responses. These data strongly suggest that BCAP plays a key role in modulating the signals that regulate NK-cell function, maturation, and survival. The data indicate that the accumulation of terminally differentiated (R3) NK cells in BCAP−/− mice is a cumulative process, which is probably the result of greater resistance to apoptosis within the mature pool of NK cells. Hayakawa and Smyth have previously noted that R3 cells are more long-lived,31,35 which is consistent with the expansion and persistence of this population in the BCAP−/− mouse.
One of the most fascinating aspects of the BCAP−/− mouse is the stark contrast between the phenotype observed in B cells and NK cells. Specifically, the opposite impacts on B cells in BCAP−/− mice are impaired ability to terminally differentiate, increased sensitivity to apoptosis, and decreased immunoglobulin production capacity.23 The differences in function and susceptibility to apoptosis can be partially explained by the higher frequency of mature NK cells and greater percentage of immature B cells, but maturation status alone is insufficient to explain the entire phenotype of BCAP−/− NK cells, as evident in the enhanced IFN-γ responses in all subsets. Accumulating evidence indicates that B cells depend on positive signaling through the BCR to promote survival and terminal differentiation.40,41 Notably, B-1 cells, which require strong BCR signaling to develop,42,43 are absent in BCAP−/− mice.23 The opposite is true for NK cells, which rely on negative signaling to reach a mature, functionally competent state.13-16 Our data indicate that BCAP is intimately involved in the contradictory development and survival of these 2 lymphocyte populations. The Akt defect that is present in NK cells from BCAP−/− mice is not present in B cells,22,23 and the calcium signaling defect in BCAP−/− B cells is not evident in NK cells (data not shown).
Two contrasting models can be proposed to explain the role of BCAP in NK-cell signaling (1) BCAP serves as a negative regulator or (2) BCAP is a positive regulator of activation signaling. Activation signals involving BCAP could derive from ITAM-linked receptors, cytokine receptors, or both. NK cells from BCAP−/− mice exhibit enhanced cytolytic function, IFN-γ production, and viral clearance responses, which supports the straightforward conclusion that BCAP is a negative regulator of activation signaling in NK cells. However, Akt signaling is significantly suppressed in BCAP−/− NK cells, indicating that BCAP acts as a positive signaling regulator to modulate NK-cell development and function.
How might the loss of a positive signaling regulator promote NK-cell development and function? Because NK cells require inhibitory receptor signaling to become functionally competent effectors,13-16 we propose that the loss of BCAP may blunt activation in a similar manner to augment this process. In line with this hypothesis, Raulet et al have proposed that the hypo-responsiveness of NK cells lacking SRIRs is caused by chronic activation signaling in the absence of inhibitory receptor engagement.13,44 Similar hyporesponsive NK cells predominate in MHC-I−/− mice or mice expressing dominant-negative SHP-1, both of which lack appropriate Ly49-mediated inhibitory signaling.17,45 Desensitization of signaling pathways is a common response to chronic ligand-mediated receptor stimulation in a variety of tissues. We propose that BCAP is involved in the desensitization pathway and the absence of BCAP may globally reduce the desensitizing activation signaling in all NK-cell subpopulations and thereby shift the balance away from hyporesponsiveness. This shift to diminished activation signaling fits a “signal strength” model, which suggests that stronger inhibitory signaling or lack of BCAP may support development and promote full functional competence, which has been termed “licensing,” “education,” or “arming” of NK cells.14,15,44,46 Therefore, the increased function, accumulation, and survival of mature NK cells in BCAP−/− mice may be the result of better damping of a desensitizing signal that is at least partially BCAP-mediated. The defect in Akt signaling seems contradictory to greater resistance to apoptosis and enhanced function in BCAP−/− NK cells, implying that compensatory mechanisms must be involved. This possibility is being investigated as a part of our ongoing studies.
The B-cell phenotype in BCAP−/− mice is similar to several other mutant mouse models, which lack other signaling proteins. In particular, a reduction in mature B-cell development and paucity of B-1 cells is observed in mice lacking BCAP, Btk, PLCγ2, BLNK, Vav, or p85α subunit of PI3-K.23,47,48 Mice deficient in PLC-γ2 or Vav isoforms have broadly hypo-responsive NK cells, however, presumably because of more profound deficits in positive signaling through these deficiencies than in BCAP−/− mice.49,50 Therefore, the effects on NK-cell development and function are unique in BCAP−/− mice, and this appears to be the result of a modest impact on activation signaling upon elimination of the signaling adaptor.
Our findings emphasize that analyses of NK cells in mutant mice should address maturation status, which can significantly influence interpretation of results. Our data further suggest that therapeutic manipulation of the BCAP pathway may be beneficial to augment NK-cell development and function while promoting apoptosis and suppressing development/function of B cells. For example, blockade of the BCAP pathway may be advantageous to enhance NK-cell development during early stages of hematopoietic stem cell transplantation in treating B-cell leukemia.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Dietmar Kappes, David Wiest, Stephen Anderson, Shahjahan Miah, Amanda Purdy, and Diana Alvarez Arias for constructive criticism and advice; research facilities at Fox Chase Cancer Center for reagents and technical support (cell culture, cancer biomarker and genotyping, hybridoma, laboratory animal facility, and cell sorting); Jim Oesterling for help with flow cytometry; Dr Eric Ross for bio-statistical analysis; Matthew Schoel, Kimberly Lauff, and Jackie Valvardi for animal care; the National Cancer Institute (NCI) Biological Resources Branch (Frederick, MD) for supplying recombinant human IL-2; and Dr Suzanne Lemieux (INRS-Institut Armand Frappier, Laval, QC, Canada) for biotinylated Anti-Ly49C.
This work was supported by grants CA083859 and CA100226 (K.S.C.), training grant AI007492 (A.W.M.), and CORE grant CA06927 from the National Institutes of Health, and an appropriation from the Commonwealth of Pennsylvania.
National Institutes of Health
Authorship
Contribution: A.W.M. performed most of the experiments, interpreted results, and contributed to writing the manuscript; T.Y. originally established the BCAP−/− mice and provided them; M.F. guided and assisted with ectromelia experiments; L.J.S. provided guidance, advice, and facilities for ectromelia experiments; T.K. originally established the BCAP−/− mice and provided them; K.S.C. supervised the project, performed some experiments, interpreted results, provided financial support, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kerry S. Campbell, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111-2497; e-mail: kerry.campbell@fccc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal