Abstract
Multiple myeloma (MM) is characterized by osteolytic bone lesions (OBL) that arise as a consequence of osteoblast inactivation and osteoclast activation adjacent to tumor foci within bone. Wnt signaling in osteoblasts regulates osteoclastogenesis through the differential activation and inactivation of Receptor Activator of Nuclear factor Kappa B Ligand (RANKL) and osteoprotegerin (OPG), positive and negative regulators of osteoclast differentiation, respectively. We demonstrate here that MM cell–derived DKK1, a soluble inhibitor of canonical Wnt signaling, disrupted Wnt3a-regulated OPG and RANKL expression in osteoblasts. Confirmed in multiple independent assays, we show that pretreatment with rDKK1 completely abolished Wnt3a-induced OPG mRNA and protein production by mouse and human osteoblasts. In addition, we show that Wnt3a-induced OPG expression was diminished in osteoblasts cocultured with a DKK1-expressing MM cell line or primary MM cells. Finally, we show that bone marrow sera from 21 MM patients significantly suppressed Wnt3a-induced OPG expression and enhanced RANKL expression in osteoblasts in a DKK1-dependent manner. These results suggest that DKK1 may play a key role in the development of MM-associated OBL by directly interrupting Wnt-regulated differentiation of osteoblasts and indirectly increasing osteoclastogenesis via a DKK1-mediated increase in RANKL-to-OPG ratios.
Introduction
Bone destruction, a cardinal feature of multiple myeloma (MM), results from an uncoupling of osteoclast and osteoblast activities adjacent to intramedullary tumor foci.1-3 Osteoclasts are activated by binding of the receptor activator of nuclear factor kappa B ligand (RANKL)416 to its cognate receptor, RANK, whereas osteoprotegerin (OPG),7 a soluble member of the tumor necrosis receptor super-family, acts as a naturally occurring decoy receptor that competes with RANK for binding of RANKL.8 The balance of these 2 molecules plays a critical role in the control of osteoclastogenesis. MM cells likely stimulate expression of RANKL and suppress expression of OPG by osteoblasts or their progenitors.9,10 Increased serum levels of RANKL and decreased levels of OPG have been associated with a poor prognosis in MM.11 Restoring the RANKL/OPG imbalance by RANKL antagonist or recombinant OPG not only reduces MM-associated bone lesions but also halts disease progression in animal models.10,12-14
Mechanistically, regulation of osteoclastogenesis by osteoblast-derived OPG15-17 and RANKL16,18,19 involves Wnt signaling, a pathway that is regulated by a large number of antagonists, including the Dickkopf family,20 secreted frizzled-related proteins (sRFPs),21,22 and sclerostin.23 DKK1 blocks maturation of osteoblasts and formation of mineralized matrix by antagonizing the canonical Wnt pathway through it binding to LRP5/6 and Kremen.24-28 Germ line inactivating mutations in the Wnt coreceptor LRP5 causes the osteoporosis-pseudoglioma syndrome (OPPG),29 whereas a high bone mass phenotype is caused by a distinctly different LRP5 mutation that prevents binding of DKK1.30,31 Overexpression of DKK1 in transgenic mice leads to decreased bone mass,27 whereas deletion of a single allele of DKK1 in mouse osteoblasts results in increased bone formation and bone mass.20 OBL in MM has been linked to DKK1 secretion by tumor cells,32-35 through a mechanism of inhibiting canonical Wnt signaling in, and differentiation of, osteoblasts.36 Blocking DKK1 with a neutralizing antibody prevents MM-induced bone resorption and tumor growth in the SCID-rab model.37
Although DKK1, RANKL, and OPG appear to play key roles in the development of OBL, whether DKK1 influences RANKL/OPG expression in MM has never been established. In the present study, we show for the first time that MM-derived DKK1 disrupts Wnt3a-induced OPG expression while releasing a Wnt-regulated block to RANKL expression in mouse and human osteoblasts, which could be effectively antagonized by anti-DKK1 neutralizing antibody. Thus, DKK1-mediated disruption of Wnt signaling in osteoblasts and their precursors may cause an imbalance in the RANKL/OPG levels in MM, providing additional rationale for the development of therapeutic strategies that target DKK1 in MM.
Methods
Primary MM cells and established MM cell lines
Primary plasma cells.
Primary plasma cells (PCs) were obtained from heparinized bone marrow (BM) aspirates from multiple myeloma (MM) patients during scheduled clinic visits. Signed University of Arkansas for Medical Sciences Institutional Review Board–approved informed consent forms, acquired in accordance with the Declaration of Helsinki, are kept on record. Mononuclear cells were isolated from BM of MM patients using a Ficoll-Hypaque density gradient centrifugation. PC isolation from mononuclear cell fraction was performed by immunomagnetic bead selection with monoclonal mouse antihuman CD138 antibodies using the AutoMACs automated separation system (Miltenyi Biotec, Auburn, CA). PC purity of more than 85% homogeneity was confirmed by 2-color flow cytometry using CD138+/CD45 and CD38+/CD45 criteria (BD Biosciences, San Jose, CA), immunocytochemistry for cytoplasm light-chain immunoglobulin (Ig), and morphology by Wright-Giemsa staining.
Cell lines.
Human MM cell line, OPM-2, was cultured in RPMI1640 as previously described.38 Mouse pluripotent mesenchymal precursor cell line C2C12 that has the potential of differentiating into osteoblast in the presence of BMP-239 and human osteoblast cell line hFOB1.19 were purchased from ATCC (Manassas, VA). C2C12 and human osteoblast-like cell line, Saos-2 and MG63 were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL), streptomycin (100 mg/mL), and 4 mM l-glutamine. Cells were maintained at 37°C and humidified with 95% air and 5% CO2 for cell culture.
Coculture system
C2C12 cells were cultured in 6-well plates in DMEM with 10% fetal bovine serum and maintained at subconfluence. MM cells (5 × 105/mL) were seeded on the C2C12 in the presence or absence of Wnt3a-CM or Cont-CM for indicated times. For coculture with primary cells, CD138+ cells were cultured on the C2C12 monolayer for 72 hours in the presence or absence of rWnt3a with and without anti-DKK1 antibody (R&D Systems, Minne-apolis, MN) for 48 hours. Total RNA was isolated using TRIZOL rea-gent (Invitrogen) for mRNA analysis. Supernatants were harvested for protein analysis.
Constructs and transfectants
A MM cell line, OPM-2, stably expressing DKK1 has been generated as previously described.40 Functional DKK1 protein was determined by blocking Wnt3a-induced TCF/LEF transcriptional activity using the TOPflash luciferase assay as previously described.40 To generate a DKK1-expressing osteoblast cell line, C2C12 cells were transfected, using Lipofectamine (Invitrogen), with a pEF-V5 vector or the same vector carrying a DKK1 cDNA, according to manufacturer's instructions. Clonal cell lines were generated by limited dilution in growth media containing blasticidin. Positive clones were detected by anti-V5 antibody (Invitrogen) with Western blotting analysis. DKK1 protein concentration in culture media of positive clones was measured by enzyme-linked immunosorbent assay (ELISA) analysis. Functional DKK1 protein was determined by analyzing the effect on stabilization of free β-catenin as previously described.36
Preparation of conditioned medium
Wnt3a conditioned medium (Wnt3a-CM) or control (Cont-CM) was prepared as previously described.40 Briefly, Wnt3a-producing L cells (stably transfected with Wnt3a cDNA kindly provided by Dr Shinji Takata) or control L cells were cultured to confluence in DMEM medium supplemented with 10% fetal calf serum after which the medium was replaced with serum-free DMEM. The culture supernatant was collected after 72 hours and designated Wnt3a-CM and Cont-CM, respectively. The concentration of Wnt3a in Wnt3a-CM was evaluated by correlating β-catenin stabilization with that of recombinant Wnt3a (R&D Systems). Comparing its effect on stabilization of β-catenin in C2C12 cells with that seen by recombinant Wnt3a was used to standardize the concentration of Wnt3a in conditioned media; 100% of Wnt3a-CM equated to 150 to 200 ng/mL of recombinant Wnt3a based on similarities in increased β-catenin levels; 50% of Wnt3a-CM was diluted with equal volume of DMEM media to treat cells. DKK1 conditioned medium (DKK1-CM) and control medium (Cont-CM) were prepared as described previously.40 DKK1 protein in DKK1-CM, Cont-CM, supernatant from culture media of C2C12, MG63, and Saos-2 cells, and sera from MM patients were measured by ELISA analysis as described previously.36
Immunoblotting analysis and GST-E-cadherin binding assay
Proteins from cell lysates derived from C2C12 and OPM-2 cells expressing DKK1 or empty vector were separated by SDS-PAGE and transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Billerica, MA). Immunoblotting was performed using the indicated antibodies as previously described.41
The GST-E-cadherin binding assay was performed as described.42 Briefly, proteins were isolated from cells that had been treated with recombinant Wnt3a for indicated times. The β-catenin binding site of E-cadherin as a GST-fusion protein was purified using GST beads. GST-E-cadherin was used to precipitate uncomplexed β-catenin in 500 mg cell lysate. Precipitated β-catenin was detected by immunoblotting analysis using a β-catenin monoclonal antibody (BD Transduction Laboratories, San Diego, CA).41
ELISA
Microtiter plates were coated with 50 μL of anti-DKK1 antibody (R&D Systems) according to manufacturer recommendations. Bone marrow serum (1:50) in dilution buffer was added and incubated overnight at 4°C. Plates were washed and incubated with biotinylated goat antihuman DKK1 IgG (R&D Systems) followed by streptavidin-horseradish peroxidase (Vector Laboratories, Burlingame, CA), according to the manufacturer's recommendations. The concentrations of OPG and RANKL proteins in cultured supernatant were measured using the kits according to the manufacturer's recommendations (R&D Systems).
Reverse transcription-PCR analysis and DNA sequence analysis
Total RNA was isolated using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed as previously described.40 All polymerase chain reaction (PCR) reactions began with a first cycle at 95°C for 3 minutes and a final cycle at 72°C for 10 minutes with an additional 35 cycles at 94°C for 30 seconds, 60°C for 45 seconds, and 72°C for 1 minute. Primers, including human and mouse DKKs, were designed using the “primer pair program” using MacVector software41 based on gene sequences from the NCBI Gene Bank (www.ncbi.nlm.nih.gov). Primer sequences and expected sizes of DNA fragments amplified for the indicated mouse and human genes are listed in Table 1. PCR fragments were subcloned using TOPO-TA cloning vector according to the manufacturer's instructions (Invitrogen) and sequence analysis performed as previously described.40 Data analysis was performed using MacVactor software and comparisons made with NCBI BLAST (http://www.ncbi.nlm.nih.gov/blast/).
DKK oligonucleotide primers for RT-PCR
| Primer . | Orientation . | Nucleotide sequence (5′ to 3′) . | Nucleotide position . |
|---|---|---|---|
| mDkk1F | Sense | ACACACACACACACACACACACATC | 1501-1525 |
| mDkk1R | Antisense | GCAAAAGCACCAACCACACTTG | 1797-1776 |
| mDkk2F | Sense | AATGCGGAAGAATGAGGGATG | 1593-1613 |
| mDkk2R | Antisense | TGCCAATCTGAAGGAAATGCC | 1839-1819 |
| mDkk3F | Sense | CGTGGACTTGGCAAAATGTAACC | 1511-1533 |
| mDkk3R | Antisense | GAGCACTGGCTTTCAGAGGTATTG | 1937-1914 |
| mDkk4F | Sense | AAGCCCCAGAAATCTTCCAGC | 697-717 |
| mDkk4R | Antisense | TGAACACAACAACAAGTCCCGTG | 839-817 |
| hDkk1F | Sense | CCAACGCGATCAAGAACCTGCC | 154-176 |
| hDkk1R | Antisense | GATGGTGATCTTTCTGTATCC | 790-811 |
| hDkk2F | Sense | CTGATGGTGGAGAGCTCACAG | 201-223 |
| hDkk2R | Antisense | CCTGATGGAGCACTGGTTTGCAG | 749-772 |
| hDkk3F | Sense | AGTACACCTGCCAGCCATG | 611-630 |
| hDkk3R | Antisense | CTCCAGGTCTTCCAGCTCCTGG | 1072-1093 |
| hDkk4F | Sense | GGTCCTGGACTTCAACAACATC | 168-190 |
| hDkk4R | Antisense | CTTAATCGAGCATGCTGCCG | 738-758 |
| Primer . | Orientation . | Nucleotide sequence (5′ to 3′) . | Nucleotide position . |
|---|---|---|---|
| mDkk1F | Sense | ACACACACACACACACACACACATC | 1501-1525 |
| mDkk1R | Antisense | GCAAAAGCACCAACCACACTTG | 1797-1776 |
| mDkk2F | Sense | AATGCGGAAGAATGAGGGATG | 1593-1613 |
| mDkk2R | Antisense | TGCCAATCTGAAGGAAATGCC | 1839-1819 |
| mDkk3F | Sense | CGTGGACTTGGCAAAATGTAACC | 1511-1533 |
| mDkk3R | Antisense | GAGCACTGGCTTTCAGAGGTATTG | 1937-1914 |
| mDkk4F | Sense | AAGCCCCAGAAATCTTCCAGC | 697-717 |
| mDkk4R | Antisense | TGAACACAACAACAAGTCCCGTG | 839-817 |
| hDkk1F | Sense | CCAACGCGATCAAGAACCTGCC | 154-176 |
| hDkk1R | Antisense | GATGGTGATCTTTCTGTATCC | 790-811 |
| hDkk2F | Sense | CTGATGGTGGAGAGCTCACAG | 201-223 |
| hDkk2R | Antisense | CCTGATGGAGCACTGGTTTGCAG | 749-772 |
| hDkk3F | Sense | AGTACACCTGCCAGCCATG | 611-630 |
| hDkk3R | Antisense | CTCCAGGTCTTCCAGCTCCTGG | 1072-1093 |
| hDkk4F | Sense | GGTCCTGGACTTCAACAACATC | 168-190 |
| hDkk4R | Antisense | CTTAATCGAGCATGCTGCCG | 738-758 |
Real-time quantitative PCR
One microgram of total RNA was reverse transcribed into total cDNA. Quantitative PCR (qPCR) was performed using an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). The primers specific for mouse OPG and RANKL or specific for human OPG and RANKL were purchased from Applied Biosystems. A reaction mixture contained 400 ng of cDNA, dedicated buffers with specific primers and probes (5′-labeled by 6-carboxy-fluorescein and 3′-labeled by 1-carboxy-teteramethyrhdamine), and DNA polymerase in a total 20 μL volume. After 2 minutes incubation at 50°C and 10 minutes incubation at 95°C for denaturing, the reaction was subjected to 40-cycle amplification at 95°C for 15 seconds to denature and at 60°C for 1 minute for annealing/extension. Each cDNA sample was analyzed in triplicate in parallel with GAPDH as a control. Changes in mRNA concentration were determined by subtracting the CT (threshold cycle) of target gene from the CT of GAPDH (Δ = CT gene − CT GAPDH). The mean of Δ control was subtracted from the Δtarget gene reaction (mean Δ control − Δtarget-gene = e). The difference was calculated as 2e by the 2-ΔΔCT.43
Silencing DKK1 expression by DKK1 short hairpin RNA
A sequence previously shown to be an effective siRNA specific to human DKK1 gene (5′-CAATGGTCTGGTACTTATTCCCGAAGGATTAAGTACCAG-ACCATTGCACC-3′)44 was used to design a synthetic double-stranded oligonucleotide sequence for short hairpin RNA (shRNA) knockdown studies, as described45 and designated shDKK1. A control oligonucleotide sequence not matching any sequence in the human genome (5′-GATCCCCGACACGCGACTTGTACCACTTCAAGAGAGTGGTACAAGTCGGTCGTCTTTTTA-3′) was used as a control shRNA sequence (designated as shCont). Both double-stranded shRNA sequences were obtained from Integrated DNA Technologies (Coralville, IA). The double-stranded oligonucleotides were cloned into pLVTH, and virus was generated by cotransfection of 293T cells with the pLVTH vector and helper plasmids pMD2G and pCMV-dR8.91 (all kindly provided by Dr Didier Trono, University of Geneva, Geneva, Switzerland). The crude lentivirus was concentrated from cultured supernatant of the 293T cells and filtered (0.45 μm), and viral titers were determined by measuring the percentage of green fluorescent protein-positive cells present 48 hours after infection of 293T cells. The Saos-2 and MG63 cells were infected with lentivirus supernatant for indicated times. The efficiency of infection with shDKK1 and shCont virus was determined by counting the percentage of green fluorescent protein-positive cells by fluorescence microscopy. Total RNA, isolated after 24, 48, or 72 hours, was subjected to reverse transcription (RT)-PCR and qPCR to determine of the degree of target gene silencing. After 72 hours after infection, supernatants of the cells were subjected to ELISA analysis to determine DKK1 protein concentration.
Statistical analysis
Statistical significance of differences between experimental groups was analyzed by a Student t test using the Microsoft Excel software statistical package (Microsoft, Redmond, WA). Significant P values were less than .05 by 2-tailed test.
Results
Wnt3a induces OPG mRNA and OPG protein levels in osteoblasts
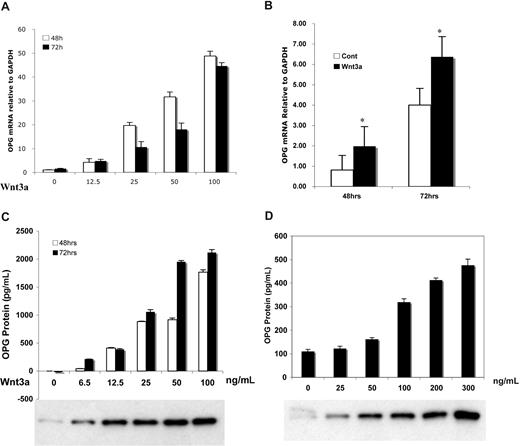
Wnt3a stimulated OPG mRNA expression in a dose-dependent and time-dependent fashion in murine mesenchymal osteoblast-precursor C2C12 cells (Figure 1A) as well as in human osteoblast-like Saos-2 cells (Figure 1B) and in MG63 cells (data not shown), increasing of 40-fold, 2-fold, and 1.5-fold, respectively. Similar results were obtained for OPG protein levels (Figure 1C), which revealed increases relative to controls by 2000-fold in C2C12 cells versus 4-fold in Saos-2 (Figure 1D). The response in changes in OPG protein levels was consistent with free β-catenin levels in the cytoplasm as measured by E-cadherin binding analysis (Figure 1C,D).
Wnt3a induced increase in OPG mRNA and protein in osteoblast progenitor cells. C2C12 cells (A,C) and Saos-2 cells (B,D) were treated with serial concentrations of recombinant Wnt3a for indicated times. The OPG mRNA (A,B) was amplified by qPCR analysis. The supernatant of treated cells (C,D) was harvested and subjected to ELISA for measurement of OPG protein. Protein in lysate (1 mg) was subjected to the GST-E-cadherin assay. After SDS-PAGE analysis, uncomplexed β-catenin was detected by anti–β-catenin antibody (C,D). The results are means plus or minus SD (n = 4). Results are representative of 3 independent experiments (*P < .05 vs control).
Wnt3a induced increase in OPG mRNA and protein in osteoblast progenitor cells. C2C12 cells (A,C) and Saos-2 cells (B,D) were treated with serial concentrations of recombinant Wnt3a for indicated times. The OPG mRNA (A,B) was amplified by qPCR analysis. The supernatant of treated cells (C,D) was harvested and subjected to ELISA for measurement of OPG protein. Protein in lysate (1 mg) was subjected to the GST-E-cadherin assay. After SDS-PAGE analysis, uncomplexed β-catenin was detected by anti–β-catenin antibody (C,D). The results are means plus or minus SD (n = 4). Results are representative of 3 independent experiments (*P < .05 vs control).
DKK1 diminishes Wnt3a-mediated OPG production in osteoblasts
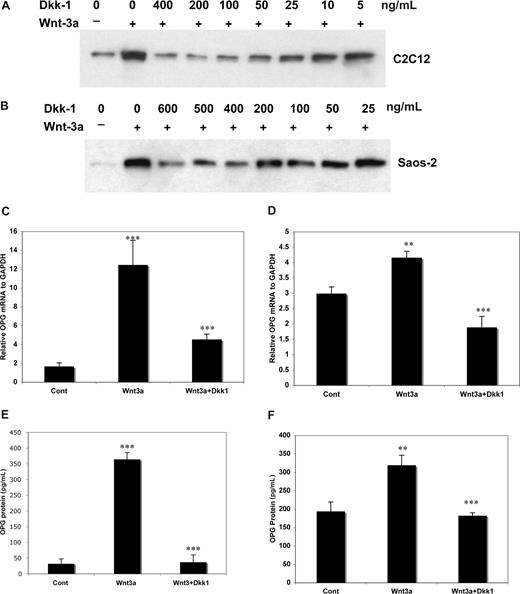
Using recombinant DKK1 protein, free β-catenin levels were reduced (using the pull-down assay) in C2C12 (Figure 2A) and in Saos-2 cells (Figure 2B). Higher DKK1 concentrations were required for effective DKK1-induced attenuation of Wnt3a-induced OPG transcription and translation in Saos-2 than C2C12 cells (Figure 2C,D). Although endogenous OPG mRNA and OPG protein levels were approximately 40-fold and 100-fold higher in Saos-2 and MG63 cells relative to C2C12 cells (Figures 1,2), induction of OPG mRNA and protein in response to Wnt3a stimulation in both Saos-2 and MG63 cells were less obvious than in C2C12 cells, suggesting a greater sensitivity of these cells to DKK1.
DKK-1 inhibition of Wnt3a induced OPG mRNA and protein in osteoblast cells. C2C12 (A) and Saos-2 (B) cells were stimulated with or without Wnt3a for 8 hours after prior treatment with recombinant DKK-1 for 1 hour at indicated concentrations and then lysed. A total of 0.5 mg of protein from cell lysates was subjected to the GST-E-cadherin assay. After SDS-PAGE, uncomplexed β-catenin was detected with anti–β-catenin antibody. The cells were cultured at 105/well in 6-well plate for 24 hours, and 100 ng/mL of Dkk1 was added for 1 hour followed by addition of 100 ng/mL of rWnt3a for 48 or 72 hours. Total RNA was isolated from treated C2C12 (C) and Saos-2 (D) cells after 48 hours and OPG mRNA was quantified. The supernatant of C2C12 (E) and Saos-2 (F) cells treated, as above for 72 hours, was harvested and subjected to ELISA for measurement of OPG. The results are shown as means plus or minus SD (n = 3). Results are representative of 3 independent experiments (**P < .01, ***P < .001, vs control).
DKK-1 inhibition of Wnt3a induced OPG mRNA and protein in osteoblast cells. C2C12 (A) and Saos-2 (B) cells were stimulated with or without Wnt3a for 8 hours after prior treatment with recombinant DKK-1 for 1 hour at indicated concentrations and then lysed. A total of 0.5 mg of protein from cell lysates was subjected to the GST-E-cadherin assay. After SDS-PAGE, uncomplexed β-catenin was detected with anti–β-catenin antibody. The cells were cultured at 105/well in 6-well plate for 24 hours, and 100 ng/mL of Dkk1 was added for 1 hour followed by addition of 100 ng/mL of rWnt3a for 48 or 72 hours. Total RNA was isolated from treated C2C12 (C) and Saos-2 (D) cells after 48 hours and OPG mRNA was quantified. The supernatant of C2C12 (E) and Saos-2 (F) cells treated, as above for 72 hours, was harvested and subjected to ELISA for measurement of OPG. The results are shown as means plus or minus SD (n = 3). Results are representative of 3 independent experiments (**P < .01, ***P < .001, vs control).
Overexpression of DKK1 in C2C12 cells reduces Wnt3-induced OPG
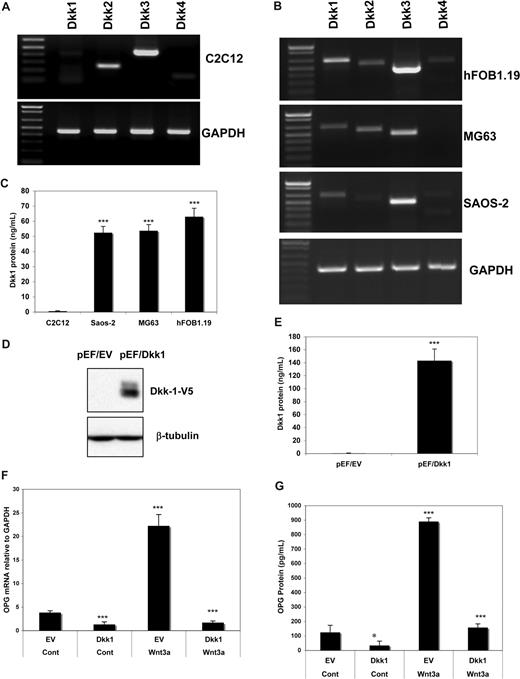
We next sought to gain mechanistic insights into the differences in response to Wnt3a stimulation relative to DKK1 concentrations required for inhibition of Wnt3a in these cell lines. We first examined the mRNA expression in OB cell lines by RT-PCR analysis. As shown in Figure 3A, Dkk1 mRNA was much weaker than Dkk2 and Dkk3 in C2C12 cells. However, relative to murine C2C12 cells, DKK1 expression was much stronger in human osteoblast lines Saos-2, MG63, and hFOB1.19 (Figure 3B). Moreover, we detected Dkk1 protein by ELISA analysis in supernatants of cultured cells at the same cell density (105/cm2) cultured for 72 hours. Consistent with the mRNA data, higher Dkk1 protein was detected in supernatants from Saos-2 and MG63 relative to that seen in C2C12 cells (Figure 3C). It should be noted that the difference in endogenous Dkk1 protein levels between human OB and C2C12 cells was more obvious than the difference in mRNA levels. These results suggest that the presence of endogenous Dkk1 protein in Saos-2 and MG63 may interfere with the cells response to Wnt3a simulation. To test this hypothesis, C2C12 cells were transfected with constructs containing Dkk1 cDNA (pEF/DKK1) or empty vector (pEF/EV), and DKK1 protein levels were detected in these stable clones by anti-V5 antibody (Figure 3D). We observed that significantly higher concentrations of DKK1 protein (160 ng/mL) in pEF/DKK1 clone supernatants were identified by ELISA compared with vector control (pEF/EV) cell supernatants (Figure 3E). OPG mRNA (Figure 3F) and OPG protein (Figure 3G) were both significantly reduced in DKK1-expressing C2C12 cells (pEF/DKK1) compared with control cells. These results suggest that murine C2C12 cells, on DKK1 transfection, become less sensitive to Wnt3a signaling and thus become more similar to the human osteoblast-like cells.
Ectopic expression of DKK1 diminished Wnt3a induced OPG mRNA and protein in osteoblast cells. The expression of DKK family members in C2C12 (A) and human osteoblast cell lines (B) as determined by RT-PCR analysis are presented. Concentration of DKK1 protein in culture supernatant of indicated cell lines by ELISA analysis (C). C2C12 cells were stable transfected with an empty vector or DKK1-expressing vector. DKK1 protein expression was detected by the anti-V5 antibody (D). DKK1 protein in the supernatant of the clones was determined by ELISA (E). The cells were treated with recombinant 100 ng/mL of rWnt3a. Relative OPG mRNA (F) and OPG protein concentration (G) was measured by qPCR or ELISA analysis as described in Figure 1. Data represent the means plus or minus SD (n = 3) of representative experiments (*P < .05, ***P < .001, and vs control).
Ectopic expression of DKK1 diminished Wnt3a induced OPG mRNA and protein in osteoblast cells. The expression of DKK family members in C2C12 (A) and human osteoblast cell lines (B) as determined by RT-PCR analysis are presented. Concentration of DKK1 protein in culture supernatant of indicated cell lines by ELISA analysis (C). C2C12 cells were stable transfected with an empty vector or DKK1-expressing vector. DKK1 protein expression was detected by the anti-V5 antibody (D). DKK1 protein in the supernatant of the clones was determined by ELISA (E). The cells were treated with recombinant 100 ng/mL of rWnt3a. Relative OPG mRNA (F) and OPG protein concentration (G) was measured by qPCR or ELISA analysis as described in Figure 1. Data represent the means plus or minus SD (n = 3) of representative experiments (*P < .05, ***P < .001, and vs control).
Silencing DKK1 by shRNA restores sensitivity to Wnt3a stimulation in Saos-2 cells
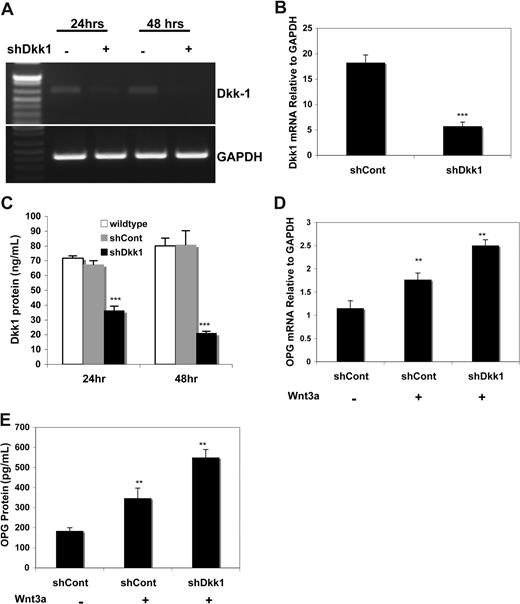
To further confirm that impaired Wnt3a signaling can be related to endogenous DKK1, DKK1-specific shRNA silencing experiments were carried out. Endogenous DKK1 mRNA in Saos-2 cells was inhibited by shDKK1, as determined by RT-PCR, but not by a nonspecific shRNA (Figure 4A). This was confirmed by qPCR (Figure 4B). Relative to shCont cells, a time-dependent significant decrease in DKK1 protein levels was observed in shDKK1-expressing Saos-2 cells (Figure 4C); such cells responded to Wnt3a treatment with a significant increase in OPG mRNA (Figure 4D) and OPG protein (Figure 4E) relative to controls. Thus, endogenous DKK1 levels control the responsiveness of Wnt3a signaling in osteoblasts as measured by OPG production.
Knockdown DKK1 by shRNA restored Wnt3a-induced OPG in osteoblasts. C2C12 cells were transiently infected with supernatant containing control siRNA (shCont) or shRNA specific for DKK1 for indicated times. Total RNA was then isolated and subjected to RT-PCR for detecting DKK1 mRNA (A). cDNA from 24 hours was subject to qPCR to confirm DKK1 mRNA expression (B). Supernatants of the cells were harvested and subjected to ELISA for measuring DKK1 protein (C). The infected cells were treated with rWnt3a for 48 hours and RNA and supernatants were harvested and subjected to qPCR and ELISA analysis for OPG mRNA (D) and protein (E). Data represent the means plus or minus SD (n = 3) of representative experiments (**P < .01, ***P < .001, vs control).
Knockdown DKK1 by shRNA restored Wnt3a-induced OPG in osteoblasts. C2C12 cells were transiently infected with supernatant containing control siRNA (shCont) or shRNA specific for DKK1 for indicated times. Total RNA was then isolated and subjected to RT-PCR for detecting DKK1 mRNA (A). cDNA from 24 hours was subject to qPCR to confirm DKK1 mRNA expression (B). Supernatants of the cells were harvested and subjected to ELISA for measuring DKK1 protein (C). The infected cells were treated with rWnt3a for 48 hours and RNA and supernatants were harvested and subjected to qPCR and ELISA analysis for OPG mRNA (D) and protein (E). Data represent the means plus or minus SD (n = 3) of representative experiments (**P < .01, ***P < .001, vs control).
Coculturing osteoblasts with MM cells expressing DKK1 prevents Wnt3a-induced OPG synthesis
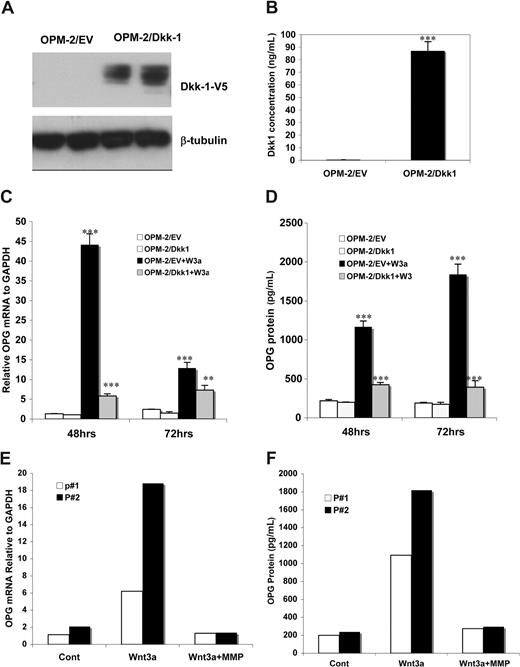
The low endogenous levels of DKK1 in C2C12 cells makes these cells particularly well suited to investigate the role of Wnt3a exposure on OPG expression in cells of the osteoblast lineage. To determine whether DKK1 expression by MM cells interferes with Wnt3a-induced OPG transcription in the BM microenvironment, the OPM-2 MM cell line stably expressing DKK1 was produced as confirmed by reduced TCF/LEF transcriptional activity relative to controls.40 Supernatants of OPM-2/DKK1 clones contained the DKK1 protein as determined by Western blot analysis with the anti-V5 antibody (Figure 5A). ELISA analysis also showed that DKK1 in OPM-2/DKK1 expressing clones was significantly higher than that seen in control cells (Figure 5B). C2C12 cells, cocultured with DKK1/OPM-2 cells, showed significant inhibition of both Wnt3a-induced OPG mRNA expression, determined by qPCR analysis (Figure 5C) and of OPG protein at 48 and 72 hours (Figure 5D).
Coculturing osteoblast cells with DKK1- expressing MM cells inhibited Wnt3a-induced OPG. A MM cell line OPM-2 was transfected with pEF6 vector (designated OPM-2/EV) or the vector containing Dkk1 cDNA (OPM-2/DKK1). DKK1 protein from OPM-2/EV or OPM-2/DKK1 cell lysates of selected clones was determined by Western blot analysis using anti-V5 antibody as described in “Immunoblotting analysis and GST-E cadherin binding assay” (A). The concentration of DKK1 protein in culture supernatants in OPM-2/EV and OPM-2/DKK1 cells was measured by ELISA analysis (B). C2C12 cells were cocultured with OPM-2/EV or OPM-2/DKK1 cells in the presence of rWnt3a or control for the indicted times. OPG synthesis in these cells, as measured by qPCR, is presented (C). Supernatants of the C2C12 were harvested and subjected to ELISA analysis to measure OPG protein concentration (D). The results are shown as means plus or minus SD (n = 4). Results are representative of 3 independent experiments (**P < .01, ***P < .001, vs control). C2C12 cells were cultured with primary CD138-positive plasma cells from 2 MM patients (P#1 and P#2) for 48 hours in the presence or absence of rWnt3a for 48 hours. The OPG mRNA in C2C12 cells was determined by qPCR (E). OPG protein levels were measured by ELISA analysis (F).
Coculturing osteoblast cells with DKK1- expressing MM cells inhibited Wnt3a-induced OPG. A MM cell line OPM-2 was transfected with pEF6 vector (designated OPM-2/EV) or the vector containing Dkk1 cDNA (OPM-2/DKK1). DKK1 protein from OPM-2/EV or OPM-2/DKK1 cell lysates of selected clones was determined by Western blot analysis using anti-V5 antibody as described in “Immunoblotting analysis and GST-E cadherin binding assay” (A). The concentration of DKK1 protein in culture supernatants in OPM-2/EV and OPM-2/DKK1 cells was measured by ELISA analysis (B). C2C12 cells were cocultured with OPM-2/EV or OPM-2/DKK1 cells in the presence of rWnt3a or control for the indicted times. OPG synthesis in these cells, as measured by qPCR, is presented (C). Supernatants of the C2C12 were harvested and subjected to ELISA analysis to measure OPG protein concentration (D). The results are shown as means plus or minus SD (n = 4). Results are representative of 3 independent experiments (**P < .01, ***P < .001, vs control). C2C12 cells were cultured with primary CD138-positive plasma cells from 2 MM patients (P#1 and P#2) for 48 hours in the presence or absence of rWnt3a for 48 hours. The OPG mRNA in C2C12 cells was determined by qPCR (E). OPG protein levels were measured by ELISA analysis (F).
The same experiment was repeated with DKK1-expressing MM plasma cells from 5 patients with newly diagnosed MM. Results were similar to those obtained with OPM-2/DKK1 cells: Wnt3a-induced OPG expression was significantly inhibited in C2C12 cells both at the mRNA (Figure 5E) and protein (Figure 5F) levels in all 5 cases. Collectively, these results suggest that DKK1-expressing MM cells impair Wnt3a-induced OPG production in osteoblasts.
Neutralization of DKK1 protein in MM sera restores OPG levels in osteoblasts
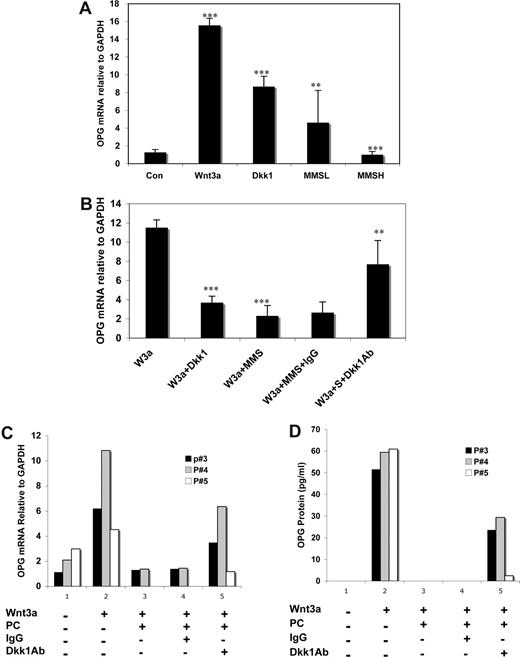
Previous studies have shown that DKK1 in sera from MM patients inhibits osteoblast differentiation32 and bone formation,33 which we have shown occurs through a DKK1-mediated attenuation of Wnt3a-induced stabilization of β-catenin.36 Similar to the presence of 100 ng/mL of rDKK1 in culture media, treatment of C2C12 cells with sera from BM of 8 MM patients containing high levels of DKK1, all in excess of 100 ng/mL of DKK1 (designated MMSH), significantly inhibited Wnt3a-induced increase in OPG mRNA (Figure 6A). The observation that sera containing less than 10 ng/mL of DKK1 protein (MMSL) still inhibited Wnt3a-induced OPG transcription might suggest that factors other than DKK1 may also contribute to interference of Wnt3a-induced OPG expression.
Neutralization of DKK1 rescued OPG expression in osteoblasts grown in the presence of MM sera or primary MM cells. C2C12 cells were treated with BM sera (50% diluted with serum free DMEM medium) from MM patients (n = 8) containing low (L; 2.7 to 8.5 ng/mL) or high (H; 104.5 to 273.5 ng/mL) concentration of DKK1 or recombinant DKK1 (100 ng/mL) as a positive control or normal sera (50% diluted with serum free DMEM medium) for 2 hours. Then rWnt3a or control vehicle was added to the cell culture media, as described above, for 48 hours (A). The cells were treated with rWnt3a or control vehicle for 48 hours after prior treatment with 25% sera from MM patients (n = 21) containing mouse Ig or anti-DKK1 antibody or with control IgG for 2 hours (B). OPG mRNA was determined by qPCR from the RNA (**P < .01, ***P < .001, vs control). Error bars represent SD. C2C12 cells were cocultured with CD138-positive plasma cells (PC) from MM or the lymphoma ST486 cell line (negative control) in the presence or absence of Wnt3a, control IgG or anti-DKK1 antibody for 48 hours. Plasma cells in suspension were removed and harvested. The supernatants were harvested by centrifugation for 10 minutes. C2C12 cells were washed with BPS and homogenized for isolation of RNA. OPG mRNA was determined by qPCR analysis from total RNA isolated from C2C12 cells (C), and OPG protein in cell culture supernatants was measured by ELISA analysis (D).
Neutralization of DKK1 rescued OPG expression in osteoblasts grown in the presence of MM sera or primary MM cells. C2C12 cells were treated with BM sera (50% diluted with serum free DMEM medium) from MM patients (n = 8) containing low (L; 2.7 to 8.5 ng/mL) or high (H; 104.5 to 273.5 ng/mL) concentration of DKK1 or recombinant DKK1 (100 ng/mL) as a positive control or normal sera (50% diluted with serum free DMEM medium) for 2 hours. Then rWnt3a or control vehicle was added to the cell culture media, as described above, for 48 hours (A). The cells were treated with rWnt3a or control vehicle for 48 hours after prior treatment with 25% sera from MM patients (n = 21) containing mouse Ig or anti-DKK1 antibody or with control IgG for 2 hours (B). OPG mRNA was determined by qPCR from the RNA (**P < .01, ***P < .001, vs control). Error bars represent SD. C2C12 cells were cocultured with CD138-positive plasma cells (PC) from MM or the lymphoma ST486 cell line (negative control) in the presence or absence of Wnt3a, control IgG or anti-DKK1 antibody for 48 hours. Plasma cells in suspension were removed and harvested. The supernatants were harvested by centrifugation for 10 minutes. C2C12 cells were washed with BPS and homogenized for isolation of RNA. OPG mRNA was determined by qPCR analysis from total RNA isolated from C2C12 cells (C), and OPG protein in cell culture supernatants was measured by ELISA analysis (D).
To verify that DKK1 in sera of MM patients contributes to the suppression of Wnt3a-mediated OPG expression in osteoblasts, MM serum was preincubated with a neutralizing antibody specific to DKK1. The experiments showed that C2C12 cells treated with Wnt3a expressed higher levels of OPG mRNA (Figure 6B) in the presence of MM serums preincubated with neutralizing DKK1 antibody relative the same MM serum treated with control nonspecific poly-Ig antibody. Furthermore, to extend our results to human CD138 primal plasma cells from 3 cases of MM patients, similar experiments were performed by pretreatment of the primal MM cells with the neutralizing antibody specific to DKK1 and then cocultured with C2C12 cells at the absence of or presence of Wnt3a using IgG as a control for Dkk1 antibody and ST486, a lymphoma cell line with well-known no-activation of Wnt signaling46 as a control for MM cells. As shown in Figure 6C, Wnt3a induced increase in OPG mRNA in C2C12 cells were significantly attenuated when cocultured with MM primal cells pretreated with control IgG, compared with control cells, and this was restored when cocultured with MM cells, which were pretreated with Dkk1 antibody. Similar results were observed in OPG protein levels in cocultured supernatant determined by ELISA analysis (Figure 6D). Collectively, these results suggest that DKK1 expressed by MM cells can negatively regulate Wnt3a-mediated OPG secretion in osteoblasts.
Wnt3a-mediated inhibition of RANKL is blocked by MM-derived DKK1
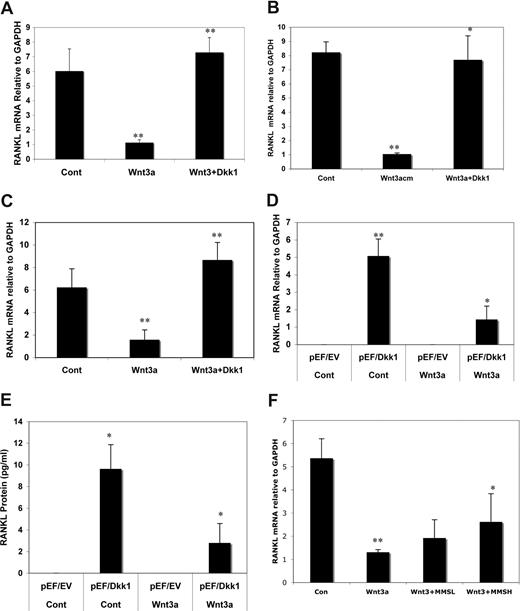
Because indirect activation of Wnt signaling by inhibition of GSK3β has been reported to regulate RANKL in MC3T3-E1 osteoblasts,19 we investigated the potential effect of DKK1 on this process as an additional mechanism underlying MM OBL. Treatment of C2C12 cells with Wnt3a for 48 hours resulted in a significant decrease in RANKL mRNA (Figure 7A), which could be restored by pretreatment of cells with rDKK1. Similar results were observed in DKK1-pretreated Saos-2 (Figure 7B) and MG63 cells (Figure 7C). To further confirm the role of DKK1 on this process, we used DKK1-overexpressing C2C12 cells, in which high DKK1 concentrations can abrogate Wnt3a signaling (Figure 3F,G). RANKL mRNA (Figure 7D) and protein expression (Figure 7E) in pEF/DKK1 cells was significantly higher than in control pEF/EV even in the absence of Wnt3a protein. Wnt3a treatment of pEF/DKK1 cells significantly altered RANKL expression (P < .01), compared with pEF/DKK1 cells without Wnt3a. These results suggest that RANKL protein production in osteoblast cells is determined by the ratio of Wnt and DKK1. We were able to show that sera from 8 subjects with MM and high DKK1 concentrations (≥ 100 ng/mL) significantly decreased Wnt3a-mediated inhibition of RANKL mRNA expression (Figure 7F), whereas sera containing low concentrations of DKK1 (< 10 ng/mL) were ineffective. Taken together, these results suggest that DKK1, through inhibition of canonical Wnt signaling, increases RANKL in osteoblasts.
DKK1 and sera from MM patients inhibits Wnt3a-induced suppression of RANKL in osteoblast. C2C12 (A), Saos-2 (B), and MG63 (C) cells were treated with rWnt3a or Wnt3a-CM (as indicated) or Cont-CM for 48 hours after prior treatment with 100 ng/mL of DKK1 protein for 2 hours. RANKL mRNA was analyzed by qPCR. C2C12 cells transfected with empty vector (pEF/EV) or the vector carrying DKK1 cDNA (pEF/DKK1) were cultured in presence or absence of rWnt3a protein (100 ng/mL). The RNA and supernatant were harvested and subjected to qPCR analysis to determine RANKL mRNA (D) or ELISA to measure RANKL protein (E). C2C12 cells were treated with rWnt3a protein for 48 hours after prior incubation with sera (50% diluted with fresh serum free DMEM medium) from MM patients (n = 8) containing low (< 10 ng/mL) or high concentration of DKK1 (> 100 ng/mL) or normal sera (50% diluted with serum free DMEM medium) for 2 hours (F). Total RNA was isolated and subjected to cDNA synthesis. RANKL mRNA was amplified by qPCR analysis. The results are shown as means plus or minus SD (n = 3; *P < .01, **P < .001, vs control).
DKK1 and sera from MM patients inhibits Wnt3a-induced suppression of RANKL in osteoblast. C2C12 (A), Saos-2 (B), and MG63 (C) cells were treated with rWnt3a or Wnt3a-CM (as indicated) or Cont-CM for 48 hours after prior treatment with 100 ng/mL of DKK1 protein for 2 hours. RANKL mRNA was analyzed by qPCR. C2C12 cells transfected with empty vector (pEF/EV) or the vector carrying DKK1 cDNA (pEF/DKK1) were cultured in presence or absence of rWnt3a protein (100 ng/mL). The RNA and supernatant were harvested and subjected to qPCR analysis to determine RANKL mRNA (D) or ELISA to measure RANKL protein (E). C2C12 cells were treated with rWnt3a protein for 48 hours after prior incubation with sera (50% diluted with fresh serum free DMEM medium) from MM patients (n = 8) containing low (< 10 ng/mL) or high concentration of DKK1 (> 100 ng/mL) or normal sera (50% diluted with serum free DMEM medium) for 2 hours (F). Total RNA was isolated and subjected to cDNA synthesis. RANKL mRNA was amplified by qPCR analysis. The results are shown as means plus or minus SD (n = 3; *P < .01, **P < .001, vs control).
Discussion
Previous studies have revealed that DKK1 secretion by MM cells may contribute to osteolysis by attenuating Wnt signaling in cells of the osteoblast lineage, which blocks their terminal differentiation. This concept is supported by the fact that, although chemotherapy-induced tumor reduction and bisphosphonate therapy is effective at halting osteolytic lesions, there is no compensatory oteoblastic or anabolic response. Results of the present study indicate, for the first time, that MM-derived DKK1 may, in addition to blocking osteoblast differentiation, contribute to the osteolytic phenotype by shifting the expression of OPG and RANKL, which are positively and negatively regulated by canonical Wnt signaling in osteoblasts, respectively. Thus, the presence of high DKK1 levels in tumor foci blocks osteoblast differentiation, which would in turn lead to reduced OPG and increased RANKL levels and an environment that would favor increased osteoclastogenesis. The evidence supporting this model are the following: (1) DKK1 inhibits Wnt3a-induced stabilization of β-catenin and reduces free-β-catenin in both mouse and human osteoblast cells, (2) exogenous administration of DKK1 or constitutive expression of DKK1 dramatically diminished Wnt3a induced OPG expression in osteoblasts, (3) silencing DKK1 expression in human osteoblast-like cells expressing endogenous DKK1 increases sensitivity and reaction to Wnt3a stimulation as determined by increases in OPG expression, (4) MM BM serum containing high DKK1 blocked Wnt3-mediated OPG expression, (5) mimicking the interaction between osteoblasts and MM cells in the BM, a coculture system also revealed that the DKK1-secreting OPM-2 MM cell line and CD138-selected BM plasma cells from patients with MM dramatically attenuated Wnt3a-induced OPG mRNA and protein production by osteoblasts, and (6) a neutralizing anti-DKK1-antibody could restore OPG expression in osteoblasts that was inhibited by the presence of MM BM serum or primary MM plasma cells. Taken together, these results support the notion that DKK1 interrupts Wnt signaling–regulated bone resorption through regulation of osteoclastogenesis by inhibiting OPG expression. Indeed, OPG levels are decreased in MM serum relative to healthy controls.47,48 The importance of OPG is evidenced by the fact that administration of recombinant OPG or OPG peptidomimetic, OP34, can inhibit bone resorption and MM-associated osteolytic bone disease in murine models.12,49 Indeed, Wnt signaling appears to indirectly inhibit osteoclastogenesis as well. We have observed that supernatants from osteoblast cells transfected with a domain negative β-catenin contain higher RANKL and lower OPG levels and that these supernatants support an increase in the derivation of human osteoclasts from CD34-postive mononuclear cells isolated from BM of MM patients, relative to control supernatant (Y.-W.Q., unpublished data, 2007). This is consistent with in vivo data that show that deletion of β-catenin results in a marked increase in osteoclast cell numbers.16 Administration of a DKK1-neutralizing antibody decreased osteoclast numbers in myelomatous bones from SCID-rab mice, perhaps through a reduction of RANKL and increase in OPG production.37
In contrast to the inhibitory effect of DKK1 on Wnt-stimulated OPG expression in osteoblast cells interacting with MM cells, DKK1 restores RANKL expression in osteoblast cells. This is supported by data showing that DKK1 significantly reversed Wnt3a-mediated downregulation of RANKL expression in mouse and human osteoblast-like cell lines and that overexpression of DKK1 in osteoblast cells and MM serum with high DKK1 levels reversed Wnt3a-mediated down-regulation of RANKL expression in these lines. These results are consistent with studies showing that DKK1 increases RANKL expression in the mouse osteoblast cell line C3H10T1/2.50 A role of Wnt signaling in the regulation of RANKL expression was first recognized by Holmen et al who reported that deletion of the Wnt inhibitory molecule APC results in an increase in RANKL expression in normal osteoblast cells in mice.16 More recently, Spencer et al illustrated that the human RANKL promoter contains TCF/LEF binding sites and overexpression of full-length β-catenin inhibits RANKL promoter activity in MC3T3-E1 cells.19 Although the source of RANKL in MM is controversial, several groups have reported a role for RANKL in MM-associated lytic lesions. RANKL is up-regulated in MM cells,9,10 and increased levels of RANKL in MM serum can be used as prognostic index in MM.11
To reach comparable levels of β-catenin stabilization, higher concentrations of Wnt3a were required in human osteoblasts relative to murine osteoblasts, which may be attributable to dramatically higher levels (∼50-fold) of endogenous DKK1 in human osteoblast lines because murine and human lines exhibit similar expression patterns of endogenous Wnt ligands and LRP5/6 coreceptor and Fz receptors.36 Consequently, ectopic constitutive expression of DKK1 in mouse C2C12 cells, which lack DKK1 expression, blocked Wnt3a-induced OPG expression to an extent similar to that seen with human osteoblast cells, which express high levels of endogenous DKK1. In contrast, knockdown of endogenous DKK1 expression in human osteoblast cells restored sensitivity to Wnt3a stimulation as exhibited by an increase in OPG expression. Thus, endogenous DKK1 in osteoblasts appears to be a key factor determining sensitivity to exogenous Wnt stimulation. The difference in DKK1 expression between these cells might reflect differences in stage of osteoblast maturation, as the mouse osteoblast progenitor cell line C2C12 represents more immature progenitor cell than the human osteosarcoma cells used in this study.39 This notion is supported by the fact that DKK1 expression is high in the late-stage osteoblast cell line KS463.28 We cannot exclude the possibility that this difference might reflect differences between mouse and humans as human BM-derived mesenchymal cells express high levels of DKK133 and DKK1 regulates human, but not mouse, mesenchymal cell differentiation into adipocytes or osteoblasts.33 The endogenous DKK1 levels in osteoblast cells should be considered an important factor when selecting these cells for studying the role of Wnt signaling in regulation in osteoblast biology.
Although Wnt3a regulates both OPG and RANKL expression and DKK1 interrupts this process, it is interesting to note that Wnt3a stimulation had stronger effects on OPG than on RANKL expression in our experiments. Wnt3a induced a pronounced increase in OPG expression in response to Wnt3a compared with its inhibitory effect on RANKL expression. In addition, although anti-DKK1 antibody restored DKK1-suppressed OPG expression, it had no effect on DKK1-mediated increase of RANKL in osteoblast cells cocultured with primary MM cells. Thus, OPG seems to be more sensitive to Wnt signaling than RANKL. However, overexpression of DKK1 and blockage of endogenous canonical Wnt signaling by expression of dominant negative β-catenin significantly increase RANKL mRNA and protein in these cells (Y.-W.Q. and J.D.S., unpublished data, 2007). Thus, it is likely that DKK1-mediated suppression of OPG, rather than its effects to increase RANKL expression, may be the more important event contributing to lytic bone destruction. However, we cannot exclude the possibility that endogenous Wnt ligands regulate OPG and RANKL and as such regulate homeostasis of osteoclastogenesis in normal physiologic conditions as osteoblast cells express many Wnt ligands.36 Another possibility that we could not address in this study was whether endogenous Wnt signaling modulates RANKL expression at levels that are below the levels of sensitivity of current methods of detection. This is supported by the fact that constitutive expression of DKK1 and lack of transcriptional activity of beta-catenin in osteoblast cells restores RANKL expression.
Mesenchymal stem cells (MSCs) give rise to osteoblasts, chondrocytes, fibroblasts, and adipocytes. It is noteworthy that Gunn et al have shown that conditioned media from MSCs can induce MM cell lines to produce DKK1 and that these MSCs also produce high levels of interleukin-6 (IL-6),51,52 a potent MM growth factor.53 Importantly, Gunn et al showed that IL-6–dependent MM cell lines proliferate in MSC conditioned media and that this growth could be inhibited when a neutralizing antibody to IL-6 is added to the cultures.51 These data suggests that MM-derived DKK1 might also contribute to tumor survival by regulating IL-6 production by MSCs.
Taken together, we propose a working hypothesis that states that MM-derived DKK1 is a key regulator of the MM tumor microenvironment that leads to secretion of MM growth factors and eventual osteolysis. DKK1-mediated inhibition of Wnt-regulated osteoblast differentiation would result in a loss of functional activity. This leads to increased expression of IL-6, an essential survival factor for MM. This block of Wnt signaling also leads to reduced OPG and an increased RANKL expression by DKK1-affected MSC or osteoprogenitor cells. In this model, we propose that the shift in the RANKL-to-OPG ratios occurring at the site of bony plasmacytomas, being propagated by high local concentrations of IL-6, results in increased local osteoclastogenesis and increased bone resorption with no anabolic response. Together, these data suggest that DKK1 represents an important therapeutically tractable target, as has been suggested by positive preclinical studies in MM37,54 and rheumatoid arthritis.55
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Stuart Rudikoff and Jeffrey Rubin, NIC, National Institutes of Health for providing us with reagents, Dr Didier Trono, University of Geneva, Geneva, Switzerland for plasmids used to construct lentiviral vector, Christopher Randolph, David R. Williams, Austin Porter III, Rachel Flinchum, and Yan Xiao at the Lambert Laboratory of Myeloma Genetics, Hong Wu from Dr Epstein's Lab for assistance, and the faculty, staff, and patients of the Myeloma Institute for Research and Therapy for their support.
This work was supported by the Senior Research Award from the Multiple Myeloma Research Foundation (Y.-W.Q.) and by grants CA97513 (J.D.S. and B.B.) and CA113992 (B.C. and J.E.) from the National Cancer Institute, the Nancy and Stephen Grand Philanthropic Fund, and the Lebow Fund for the Cure of Myeloma.
National Institutes of Health
Authorship
Contribution: Y.-W.Q. conceptualized and designed the research, designed and performed the experiments, analyzed and interpreted the results, made figures, and wrote the paper; Y.C., N.B., and O.S. performed experiments; B.C. and J.E. prepared shRNA constructs and lentivirus supernatant; B.B. provided clinical material and clinic data; J.D.S. conceptualized and designed the research, analyzed and interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: J.D.S., Y.-W.Q., and B.B. have filed patents, and have licensing agreements, related to DKK1 and myeloma bone disease. The other authors declare no competing financial interests.
Correspondence: John D. Shaughnessy Jr, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4301 West Markham St, Little Rock, AR 72205; e-mail: shaughnessyjohn@uams.edu; or Ya-Wei Qiang, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4301 West Markham St, Little Rock, AR 72205; e-mail: YQiang@uams.edu.