Abstract
The small GTP-binding protein Ral has been implicated in regulated exocytosis via its interaction with the mammalian exocyst complex. We have previously demonstrated that Ral is involved in exocytosis of Weibel-Palade bodies (WPBs). Little is known about intracellular signaling pathways that promote activation of Ral in response to ligand binding of G protein–coupled receptors. Here we show that RNAi-mediated knockdown of RalGDS, an exchange factor for Ral, results in inhibition of thrombin- and epinephrine-induced exocytosis of WPBs, while overexpression of RalGDS promotes exocytosis of WPBs. A RalGDS variant lacking its exchange domain behaves in a dominant negative manner by blocking release of WPBs. We also provide evidence that RalGDS binds calmodulin (CaM) via an amino-terminal CaM-binding domain. RalGDS association to CaM is required for Ral activation because a cell-permeable peptide comprising this RalGDS CaM-binding domain inhibits Ral activation and WPB exocytosis. Together our findings suggest that RalGDS plays a vital role in the regulation of Ral-dependent WPB exocytosis after stimulation with Ca2+- or cAMP-raising agonists.
Introduction
Weibel-Palade bodies (WPBs) are endothelial cell-specific storage organelles1 that contain a number of hormones, chemokines, enzymes, and adhesive molecules that are rapidly released or recruited to the cell surface upon stimulation with specific agonists. Its main constituent, von Willebrand factor (VWF),2 is also the driving force for the biogenesis of WPBs from the trans-Golgi network.3-5 Current evidence suggests that VWF provides a platform for the cosegregation of a number of other bioactive compounds that include P-selectin, angiopoietin-2, and the chemokines interleukin-8 (IL-8) and eotaxin-3 into this intracellular storage compartment.6 Release or surface presentation of WPB constituents enables the endothelium to control vascular homeostasis, by participating in diverse processes such as the arrest of bleeding, inflammatory responses, and angiogenesis
Regulation of WPB exocytosis by both Ca2+- and cAMP-raising agonists involves signaling pathways initiated by agonist binding to G protein–coupled receptors (GPCRs) that ultimately result in fusion of WPBs with the plasma membrane.7 Real-time imaging of the secretory behavior has shown that rapid release of WPBs is observed after stimulation of endothelial cells with Ca2+-raising agonists such as thrombin or histamine.8,9 Stimulation of endothelial cells with agents that raise intracellular cAMP, such as epinephrine or vasopressin, promote a relatively slow release of WPBs, whereas a subpopulation of WPBs escapes cAMP-mediated exocytosis by clustering at the microtubule organizing center (MTOC).8,10,11
We previously reported that the small GTP-binding protein RalA cosediments with WPBs on density gradients and multiple lines of evidence suggest that activation of Ral is a crucial step in both thrombin- and epinephrine-induced exocytosis of WPBs.12-14 Ral has been implicated in regulated release of secretory granules of various origins,15-18 playing a dual role in the process of exocytosis. Ral is involved in tethering secretory vesicles to specific sites on the plasma membrane through its GTP-dependent interaction with components of the exocyst complex.16 In addition, Ral modulates exocytosis by enhancing ADP-ribosylation factor 6 (ARF6)–dependent phospholipase D1 activity,19 resulting in the formation of fusogenic lipids that promote membrane fusion.
The guanine exchange factor (GEF) involved in the activation of RalA in response to GPCR-mediated signaling in endothelial cells has not been identified. Recently, the activity of the RalGEF Ral-GDP dissociation stimulator (RalGDS) was found to be regulated by GPCR activation via receptor activation-mediated dissociation of RalGDS/β-arrestin complexes.20 In this study, we investigated whether RalGDS is involved in Ral-mediated exocytosis of WPBs. We report here that siRNA-mediated knockdown of RalGDS markedly inhibited thrombin- and epinephrine-induced WPB exocytosis. Furthermore, a RalGDSΔ382-597 mutant lacking the catalytic exchange domain was found to act in a dominant negative fashion by inhibiting exocytosis of WPBs. Previously, we observed that activation of Ral by thrombin was delayed in the presence of inhibitors of calmodulin. We now provide evidence that calmodulin binds to RalGDS via an amino-terminal CaM-binding site. A cell-permeable peptide comprising this CaM-binding motif was found to prevent thrombin-induced exocytosis of WPBs by inhibiting the activation of Ral. Together our results indicate that RalGDS plays a central role in the Ral-mediated, stimulus-induced exocytosis of WPBs from endothelial cells.
Methods
Reagents and antibodies
Culture media, trypsin, penicillin, streptomycin, and oligofectamine were from Invitrogen (Breda, The Netherlands). Thrombin, endothelial cell growth supplement (ECGS), heparin, epinephrine, IBMX, and anti–α-tubulin monoclonal antibody (DM1A) were from Sigma-Aldrich Chemie (Steinheim, Germany). Anti-Ral and anti-GFP (JL-8) monoclonal antibodies were from BD Transduction Laboratories (Lexington, KY). Anti–β-catenin (sc-7199) and anti-RalGDS (sc-25636) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-VWF monoclonal antibody CLB-RAg35 has been described previously.21 Texas red–conjugated horse anti–mouse IgG and Vectaschield mounting medium were obtained from Vector Laboratories (Burlington, VT). Alexa488- and Alexa594-conjugated goat anti–mouse IgG and goat anti–rabbit IgG secondary antibodies and Alexa568-conjugated phalloidin were from Molecular Probes (Breda, The Netherlands). Calmodulin (CaM)–agarose was from Stratagene (Amsterdam, The Netherlands). Glutathione sepharose and streptavidin sepharose were purchased from GE Healthcare (Little Chalfont, United Kingdom). The RalGDS-derived peptide TAT-CaMBD was synthesized using Fmoc (N-(9-fluorenyl)methoxycarbonyl) solid-phase chemistry and corresponds to the following sequence: biotin-YARAAARQARAGVTYSISLRKVQLHHGGNKGQRWL, in which the sequence in italics represents the TAT sequence and the sequence in bold represents the putative CaMBD of RalGDS, separated by a glycine linker. Chemiluminescence blotting substrate and Complete Protease Inhibitor Cocktail Tablets were from Roche Diagnostics (Mannheim, Germany). All chemicals used were of analytic grade.
Cell culture
HEK293 cells were maintained in DMEM-F12 medium supplemented with 10% FCS, 100 units/mL penicillin, and 100 μg/mL streptomycin. Transiently transfected HEK293 cells were obtained by electroporation using an AMAXA nucleofector in combination with the V5 cell-line nucleofector kit (AMAXA, Cologne, Germany). After transfection, cells were grown for 24 to 48 hours prior to further experiments. Endothelial cells were isolated from umbilical veins and cultured in medium containing M199, 20% fetal calf serum, 100 units/mL penicillin, 100 mg/L streptomycin, 33 mg/L l-glutamine, 12.5 mg/L ECGS, and 50 mg/mL heparin. Human umbilical vein endothelial cells (HUVECs) were transfected by electroporation using an AMAXA nucleofector in combination with the basic nucleofector kit for primary human endothelial cells (AMAXA). After transfection, HUVECs were grown on glass coverslips for 48 hours. Stimulation of endothelial cells with thrombin was performed in the following manner: HUVECs were washed twice with culture medium in which the fetal calf serum was replaced by 1% human albumin (serum-free [SF] medium). After washing, the cells were preincubated with SF medium for 1 hour. At the beginning of stimulation, the preincubation medium was replaced by SF medium containing 1 U/mL thrombin or 10 μM epinephrine and 100 μM IBMX. After stimulation, cells were fixed in 3.7% formaldehyde for 10 minutes. WPBs were visualized using CLB-RAg35 in combination with Texas red–conjugated horse anti–mouse IgG or Alexa488-conjugated goat anti–mouse IgG as secondary antibodies diluted in PBS; 1% BSA; 0.02% saponin. Where applicable, cellular membranes were visualized using anti–β-catenin and Alexa594-conjugated goat anti–rabbit IgG. Cells were embedded in Vectashield mounting medium and analyzed by confocal microscopy using a Zeiss LSM510 (Carl Zeiss, Jena, Germany) using a Plan Apochromat 63×/1.40 NA oil objective and appropriate filters. Images were generated by making optical sections (Z-stacks with 0.36-μm intervals). Z-stacks of single cells were analyzed using Image Pro Plus 6.0 (Media Cybernetics, Breda, The Netherlands) to quantify the total number of WPBs in a single cell (as delineated by β-catenin staining). Differences in WPB numbers were statistically analyzed using a Student t test.
siRNA and DNA constructs
A pool of 4 siRNA oligonucleotides directed against RalGDS (ON-TARGETplus no. J-005193) was purchased from Dharmacon (Lafayette, IN). A nontargeting siRNA (ON-TARGETplus) was used as a control in these experiments. siRNA (100 pmol) was delivered to HUVECs in 2 consecutive transfections using Oligofectamine according to the manufacturer's instructions. Alternatively, siRNA was delivered to HUVECs in 2 steps: initially, approximately 250 000 HUVECs were nucleofected with 50 pmol siRNA oligos using an AMAXA nucleofector. A second transfection with 100 pmol siRNA oligos was performed 48 hours later using Dharmafect 1 transfection reagent (Dharmacon) according to the manufacturer's instructions. Cells were left for 48 hours before assaying. Both methods resulted in similar transfection efficiencies and phenotypes. GFP-RalA was constructed by cloning a KpnI-BamHI RalA polymerase chain reaction (PCR) fragment into the KpnI/BamHI cut pEGFP-C1 vector from Clontech (BD Biosciences Europe, Erembodegem, Belgium). The GFP-RalGDS construct and the GFP-RalGDSΔ382-597 variant have been described previously.20
Ral activation assay
The amount of Ral that is activated upon stimulation was measured in a pull-down assay. The Ral-binding domain (RalBD) of the putative Ral effector RalBP1/RLIP76 fused to a GST tag was expressed in IPTG-induced bacteria as described previously.22 Purified GST-RalBD (100 μg/sample) was precoupled to 30 μL/sample of glutathione sepharose for 1 hour at 4°C. The precoupled glutathione sepharose was then washed 3 times with lysis buffer containing 15% (vol/vol) glycerol, 1% NP-40, 50 mM Tris (pH 7.5), 200 mM NaCl, 2.5 mM MgCl2, 10 mM benzamidine, 100 nM aprotinin, supplemented with 1 protease inhibitor tablet per 50 mL. Cells, grown in 6-wells plates, were lysed in 400 μL lysis buffer. The activated, GTP-bound form of Ral was then isolated from cell lysates by incubation of 300 μL lysate with the RalBD precoupled glutathione sepharose for 1 hour at 4°C. Finally, the sepharose beads were washed 4 times with lysis buffer and analyzed on a 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel by Western blotting with anti-RalA monoclonal antibody.
CaM pull-down assays
For the CaM pull-down assays, transfected HEK293 or untransfected HUVECs, grown in 6-well plates for 24 hours were lysed in 1 mL ice-cold CaM-PD lysis buffer/well containing 50 mM Tris (pH 8.0), 150 mM NaCl, 2 mM CaCl2, 1 mM MgAc2, 1 mM imidazole, 1% Triton X-100, and 1 protease inhibitor tablet per 50 mL. Lysate (900 μL) was incubated with 100 μL CaM-agarose for 1 hour at 4°C. Subsequently, CaM-agarose was washed 4 times with CaM-PD lysis buffer and analyzed for the presence of GFP-RalGDS or endogenous RalGDS on a 7.5% SDS-PAGE gel by Western blotting with an anti-GFP (JL-8) monoclonal antibody or an anti-RalGDS polyclonal antibody, respectively. For the TAT-peptide pulldown from HUVEC lysate, 250 μg biotin-tagged TAT, TAT-Ral-c, or TAT-CaMBD was coupled to 50 μL streptavidin sepharose for 1 hour at 4°C. The precoupled streptavidin sepharose was then washed 3 times with CaM-PD lysis buffer. HUVECs were grown in 80-cm2 flasks until confluence and then lysed in 1 mL CaM-PD lysis buffer/flask. HUVEC lysate (900 μL) was incubated with the precoupled streptavidin sepharose for 1 hour at 4°C, washed 4 times with CaM-PD lysis buffer, and analyzed for the presence of CaM on a 12.5% SDS-PAGE gel by Western blotting with an anti-CaM monoclonal.
Results
RalGDS knockdown reduces stimulus-induced WPB exocytosis
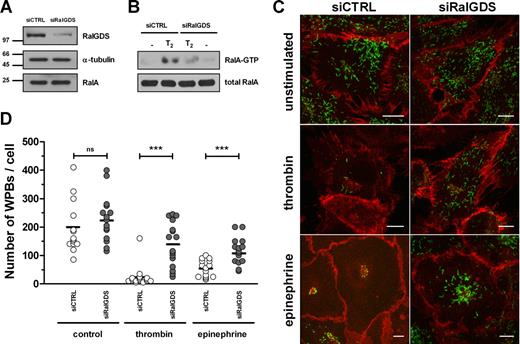
Expression of wild-type RalA and the constitutive active mutant RalA-G23V resulted in secretagogue-independent loss of WPBs, while the dominant negative mutant RalA-S28N had no effect on WPB numbers.13 In addition, a RalA-derived, carboxy-terminal peptide has been found to inhibit both thrombin- and epinephrine-induced VWF secretion by inhibition of WPB exocytosis,14 which implicates the carboxy-terminal hypervariable domain of RalA in the binding of crucial factors regulating this process. These previous results suggest that exocytosis of WPBs requires the activation of RalA. Small GTPases are activated by GEFs that induce GDP release and thus enhance GTP binding to the GTPase. A possible candidate responsible for the activation of Ral in endothelial cells is RalGDS, a well-studied, widely expressed exchange factor for Ral.23 Activation of a G protein–coupled receptor (GPCR) following ligand binding results in the dissociation of inactive RalGDS/β-arrestin complexes enabling RalGDS to activate Ral.20 Because both thrombin and epinephrine induce WPB exocytosis via stimulation of the GPCRs protease-activated receptor 1 (PAR 1) and β2-adrenergic receptor (β2-AR), respectively, we investigated whether RalGDS is involved in WPB exocytosis in HUVECs. First, we determined whether HUVECs express RalGDS by performing a RalGDS-specific PCR on cDNA acquired from 3 different HUVEC donors. These experiments showed expression of RalGDS mRNA in all 3 HUVEC samples (not shown). These results confirm gene expression data from the NCBI Gene Expression Omnibus (GEO)24 that RalGDS is expressed in HUVECs. To investigate whether RalGDS plays a role in exocytosis of WPBs, we down-regulated RalGDS expression in HUVECs using RNA interference. Small interfering RNAs (siRNAs) that target RalGDS (siRalGDS) were able to specifically knock down the expression of RalGDS by more than 80% compared with endothelial cells that were treated with a control siRNA (siCTRL) (Figure 1A). Down-regulation of RalGDS inhibited HUVECs in their ability to activate Ral in response to thrombin: thrombin-induced activation of RalA was approximately 70% reduced in HUVECs treated with siRalGDS compared with siCTRL-treated cells (Figure 1B). We questioned whether knockdown of RalGDS in HUVECs is also able to inhibit stimulus-induced exocytosis of WPBs. HUVECs were transfected with siRalGDS or siCTRL and were subsequently stimulated with 1 U/mL thrombin or 10 μM epinephrine and 100 μM IBMX, respectively. Analysis of the number of WPBs per cell revealed a loss of WPBs upon thrombin and epinephrine stimulation in cells treated with siCTRL (unstimulated: 199.4 ± 93.1 [n = 13]; thrombin: 24.7 ± 37.9 [n = 15]; epinephrine: 54.5 ± 30.3 [n = 15]; Figure 1C,D), similar to that observed previously in untransfected HUVECs. The number of WPBs in unstimulated HUVECs was not significantly different after knockdown of RalGDS (unstimulated: 228.5 ± 82.9 [n = 15]). However, in HUVECs treated with siRalGDS, the number of remaining WPBs upon thrombin or epinephrine stimulation was significantly higher compared with siCTRL-treated cells (thrombin: 140.3 ± 77.2 [n = 15]; epinephrine: 116.3 ± 47.0 [n = 15]; Figure 1C,D), indicating that down-modulation of RalGDS results in inhibition of stimulus-induced WPB exocytosis.
RalGDS knockdown by siRalGDS impairs stimulus-induced exocytosis of WPBs. (A) HUVECs were treated in 2 consecutive transfection rounds with a pool of 4 siRNA oligonucleotides directed against RalGDS (siRalGDS) or a control siRNA oligonucleotide (siCTRL). Western blot analysis 48 hours after transfection showed down-regulation of RalGDS expression, while α-tubulin and RalA remained unaffected. (B) Activation of RalA in siCTRL- and siRalGDS-treated HUVECs in response to thrombin was determined using a Ral-GTP specific pulldown. Cells were preincubated with SF medium for 1 hour, after which they were stimulated for 2 minutes with 1 U/mL thrombin (T2) or SF medium alone (−). All lysates contained similar amounts of RalA (bottom panel). (C) Representative confocal images of siCTRL- and siRalGDS-treated HUVECs that were incubated for 45 minutes with 1 U/mL thrombin, 10 μM epinephrine, and 100 μM IBMX or SF medium alone (unstimulated). WPBs were visualized by immunofluorescent staining of VWF (green), while staining of β-catenin (red) was used to delineate the cell membrane. Bars correspond to 10 μm. (D) Numbers of WPBs in individual cells were quantified as described in “CaM pull-down assays.” Approximately 15 randomly selected cells were counted for each experimental condition. Shown is the analysis of 1 experiment representative of 3 independent experiments. Horizontal bars represent medians. ***P < .001 by Student t test.
RalGDS knockdown by siRalGDS impairs stimulus-induced exocytosis of WPBs. (A) HUVECs were treated in 2 consecutive transfection rounds with a pool of 4 siRNA oligonucleotides directed against RalGDS (siRalGDS) or a control siRNA oligonucleotide (siCTRL). Western blot analysis 48 hours after transfection showed down-regulation of RalGDS expression, while α-tubulin and RalA remained unaffected. (B) Activation of RalA in siCTRL- and siRalGDS-treated HUVECs in response to thrombin was determined using a Ral-GTP specific pulldown. Cells were preincubated with SF medium for 1 hour, after which they were stimulated for 2 minutes with 1 U/mL thrombin (T2) or SF medium alone (−). All lysates contained similar amounts of RalA (bottom panel). (C) Representative confocal images of siCTRL- and siRalGDS-treated HUVECs that were incubated for 45 minutes with 1 U/mL thrombin, 10 μM epinephrine, and 100 μM IBMX or SF medium alone (unstimulated). WPBs were visualized by immunofluorescent staining of VWF (green), while staining of β-catenin (red) was used to delineate the cell membrane. Bars correspond to 10 μm. (D) Numbers of WPBs in individual cells were quantified as described in “CaM pull-down assays.” Approximately 15 randomly selected cells were counted for each experimental condition. Shown is the analysis of 1 experiment representative of 3 independent experiments. Horizontal bars represent medians. ***P < .001 by Student t test.
Dominant negative RalGDSΔ382-597 blocks stimulus-induced WPB exocytosis
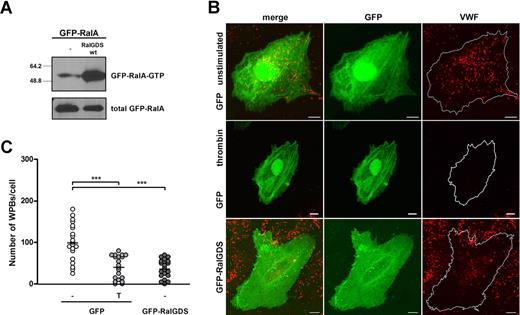
Subsequently, we determined the effect of expression of RalGDS, which was tagged with GFP to allow for easy identification of transfected cells, on RalA activation and WPB exocytosis. First, we assessed whether GFP-RalGDS could enhance the activation of RalA in vivo. Expression of GFP-RalGDS in HEK293 cells clearly stimulated activation of exogenous cotransfected GFP-RalA in HEK293 cells (Figure 2A). Furthermore, GFP-RalGDS expression in HUVECs resulted in the formation of filopodia, shown by actin costaining, which is reminiscent of RalA activation25 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The extent of filopodia formation was similar to that observed upon overexpression of GFP-RalA in HUVECs, whereas no filopodia were observed in HUVECs transfected with GFP alone. To study the effect of RalGDS overexpression on WPB exocytosis, HUVECs were transfected with GFP-RalGDS or GFP alone, and the number of intracellular WPBs in GFP-positive cells was quantified. Expression of GFP-RalGDS resulted in a reduction in the number of WPBs per cell, similar to the one observed in GFP-expressing cells after stimulation with thrombin (Figure 2B,C). Stimulation of GFP-RalGDS–expressing cells with thrombin did not result in a further decrease in the number of WPBs (data not shown). These results suggest that overexpression of RalGDS promotes exocytosis of WPB. However, it may also indicate that overexpression of RalGDS inhibits the formation of WPBs. To more specifically address the involvement of RalGDS in RalA-regulated release, we determined the effect of the dominant negative GFP-RalGDSΔ382-597 mutant, which lacks the catalytic exchange domain (Figure 3A), on RalA activation and WPB exocytosis. Expression of GFP-RalGDSΔ382-597 in HEK293 cells did not result in activation of RalA, in contrast to expression of full-length GFP-RalGDS (Figure 3B). Next, we investigated whether GFP-RalGDSΔ382-597 was able to block stimulus-induced exocytosis. In HUVECs, GFP-RalGDSΔ382-597 expression markedly reduced thrombin- and epinephrine-induced degranulation (Figure 3C), which suggests that GFP-RalGDSΔ382-597 is able to act as a dominant negative in stimulus-induced WPB exocytosis (Figure 3C). These results indicate that the exchange factor RalGDS regulates stimulus-induced exocytosis of WPBs through the activation of Ral.
RalGDS induces RalA activation and WPB exocytosis. (A) Activation of GFP-RalA upon coexpression of GFP-RalGDS in HEK293 cells was determined using a Ral-GTP–specific pulldown. Total GFP-RalA levels are shown in the lower panel showing equal expression. (B) HUVECs were transfected with GFP (negative control) or GFP-RalGDS and grown for 48 hours. GFP-expressing cells were incubated with 1 U/mL thrombin (T) or SF medium alone (−) for 45 minutes. WPBs were visualized by immunofluorescent staining of VWF (red). Shown are representative confocal images of HUVECs expressing GFP or GFP-RalGDS. Bars correspond to 10 μm. (C) The numbers of WPBs in individual, randomly selected GFP- and GFP-RalGDS–positive cells were quantified using confocal microscopy. Horizontal bars represent medians. ***P < .001 by Student t test.
RalGDS induces RalA activation and WPB exocytosis. (A) Activation of GFP-RalA upon coexpression of GFP-RalGDS in HEK293 cells was determined using a Ral-GTP–specific pulldown. Total GFP-RalA levels are shown in the lower panel showing equal expression. (B) HUVECs were transfected with GFP (negative control) or GFP-RalGDS and grown for 48 hours. GFP-expressing cells were incubated with 1 U/mL thrombin (T) or SF medium alone (−) for 45 minutes. WPBs were visualized by immunofluorescent staining of VWF (red). Shown are representative confocal images of HUVECs expressing GFP or GFP-RalGDS. Bars correspond to 10 μm. (C) The numbers of WPBs in individual, randomly selected GFP- and GFP-RalGDS–positive cells were quantified using confocal microscopy. Horizontal bars represent medians. ***P < .001 by Student t test.
GFP-RalGDSΔ382-597 blocks stimulus-induced WPB exocytosis. (A) Schematic representation of GFP-tagged RalGDS and RalGDSΔ382-597, which lacks the catalytic CDC25 domain. (B) RalA activation upon coexpression of GFP-RalGDS or GFP-RalGDSΔ382-597 with GFP-RalA was determined using a Ral-GTP–specific pulldown. Middle and bottom panels show total GFP-RalA, and GFP-RalGDS and GFP-RalGDSΔ382-597, respectively, to confirm equal expression. A vertical line has been inserted to indicate a repositioned gel lane. (C) HUVECs were transfected with GFP (negative control) or GFP-RalGDSΔ382-597 and grown for 48 hours. Cells were incubated with 1 U/mL thrombin, 10 μM epinephrine, and 100 μM IBMX or SF medium alone (unstimulated) for 45 minutes. WPB numbers in GFP- and GFP-RalGDSΔ382-597–positive cells were quantified using confocal microscopy. Horizontal bars represent medians. ***P < .001 by Student t test.
GFP-RalGDSΔ382-597 blocks stimulus-induced WPB exocytosis. (A) Schematic representation of GFP-tagged RalGDS and RalGDSΔ382-597, which lacks the catalytic CDC25 domain. (B) RalA activation upon coexpression of GFP-RalGDS or GFP-RalGDSΔ382-597 with GFP-RalA was determined using a Ral-GTP–specific pulldown. Middle and bottom panels show total GFP-RalA, and GFP-RalGDS and GFP-RalGDSΔ382-597, respectively, to confirm equal expression. A vertical line has been inserted to indicate a repositioned gel lane. (C) HUVECs were transfected with GFP (negative control) or GFP-RalGDSΔ382-597 and grown for 48 hours. Cells were incubated with 1 U/mL thrombin, 10 μM epinephrine, and 100 μM IBMX or SF medium alone (unstimulated) for 45 minutes. WPB numbers in GFP- and GFP-RalGDSΔ382-597–positive cells were quantified using confocal microscopy. Horizontal bars represent medians. ***P < .001 by Student t test.
RalGDS interacts with calmodulin
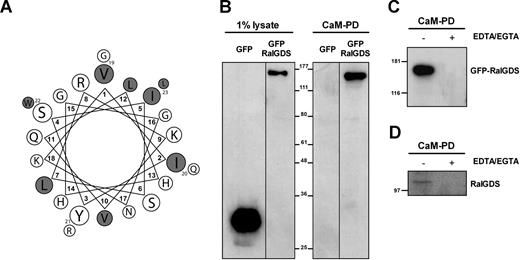
Previous work from our laboratory revealed that thrombin-induced RalA activation is inhibited by the Ca2+-chelator BAPTA-AM (M.G.R., unpublished observations, May 2002) and the CaM inhibitor trifluoperazine (TFP).13 Binding of Ca2+/CaM to the C-terminus of RalA has been shown to enhance RalA activation by 2- to 3-fold,26 however not to the extent observed in response to thrombin, suggesting that the combined action of CaM and a RalGEF is required for maximal activation of RalA. Here, we addressed whether RalGDS interacts with CaM during thrombin-induced exocytosis of WPBs. To investigate this possibility in more detail, we analyzed the amino acid sequence of the RalGDS protein for the presence of a CaM-binding site using the Calmodulin Target Database.27,28 A putative IQ-like CaMBD was found in the N-terminal region of RalGDS at position 75-97 (VIYSISLRKVQLHHGGNKGQRWL). This amino acid sequence contains 8 hydrophobic residues (VIILVLWL) of which 4 are located on one side of the helical wheel displaying the characteristic features of an amphiphilic helix (Figure 4A). To investigate whether RalGDS is indeed able to bind CaM, CaM-agarose beads were used to pull down lysates of HEK293 transfected with GFP (control) or GFP-RalGDS. Western blot analysis using an anti-GFP antibody showed that GFP-RalGDS was bound to the CaM-agarose, whereas GFP alone did not (Figure 4B). As expected, no binding of GFP-RalGDS to CaM was observed in the presence of 5 mM EDTA or EGTA (Figure 4C), indicating that the interaction between RalGDS and CaM is Ca2+ dependent. Complementary to this finding, we found that CaM-agarose was able to pull down endogenous RalGDS from endothelial cells, also in a Ca2+-dependent manner (Figure 4D).
RalGDS binds CaM in a Ca2+-dependent manner. (A) A putative calmodulin-binding domain (CaMBD) was identified in the amino-terminal region of RalGDS (aa's 75-97). An axial helical projection was generated from this amino acid sequence as outlined in the calmodulin target database.27 Hydrophobic residues are shown in gray. (B) CaM pulldown (CaM-PD) from lysates of HEK293 cells expressing GFP or GFP-RalGDS using CaM-agarose as described in “CaM pull-down assays.” The CaM-agarose specifically pulled down GFP-RalGDS. One percent of the total lysate was used to show expression levels (left). Vertical lines have been inserted to indicate repositioned gel lanes. (C) GFP-RalGDS binding to CaM is Ca2+ dependent, as the presence of 5 mM EDTA/EGTA completely abolished this interaction. (D) CaM pulldown of endogenous RalGDS from HUVEC lysates. Endogenous RalGDS was precipitated by CaM-agarose in a Ca2+-dependent manner.
RalGDS binds CaM in a Ca2+-dependent manner. (A) A putative calmodulin-binding domain (CaMBD) was identified in the amino-terminal region of RalGDS (aa's 75-97). An axial helical projection was generated from this amino acid sequence as outlined in the calmodulin target database.27 Hydrophobic residues are shown in gray. (B) CaM pulldown (CaM-PD) from lysates of HEK293 cells expressing GFP or GFP-RalGDS using CaM-agarose as described in “CaM pull-down assays.” The CaM-agarose specifically pulled down GFP-RalGDS. One percent of the total lysate was used to show expression levels (left). Vertical lines have been inserted to indicate repositioned gel lanes. (C) GFP-RalGDS binding to CaM is Ca2+ dependent, as the presence of 5 mM EDTA/EGTA completely abolished this interaction. (D) CaM pulldown of endogenous RalGDS from HUVEC lysates. Endogenous RalGDS was precipitated by CaM-agarose in a Ca2+-dependent manner.
Cell-permeable peptide corresponding to the putative CaMBD of RalGDS inhibits thrombin-induced WPB exocytosis
To further investigate the role of the putative CaM-binding domain (CaMBD) of RalGDS, we designed a cell-permeable peptide corresponding to amino acids 75 to 97 of the RalGDS protein. In addition to the putative CaMBD, this peptide contains the protein transduction domain (TAT) of human immunodeficiency virus, which enables the peptide to be transduced directly into the cell.29 To see whether the TAT-CaMBD peptide could bind to CaM, we performed pull-down experiments from HUVEC lysate. As a control we used a TAT-Ral-c peptide containing the carboxy-terminal 26 amino acids of RalA which is known to bind to CaM and a peptide containing only the TAT transduction domain. Western blot analysis using a monoclonal anti-CaM antibody showed that TAT-CaMBD is indeed able to bind endogenous CaM from HUVEC lysate (Figure 5A). As expected, also the TAT-Ral-c peptide was capable of binding to CaM, although to a lesser extent, whereas no CaM binding was observed with the TAT peptide.
A cell-permeable peptide comprising the N-terminal CaMBD of RalGDS inhibits thrombin-induced WPB exocytosis. (A) The biotin-labeled peptides TAT-CaMBD, TAT (negative control), and TAT-Ral-c (positive control) were coupled to streptavidin-sepharose and used in a pull-down experiment from HUVEC lysate as described in “CaM pull-down assays.” The bottom panel shows total CaM levels to show equal expression. (B) RalA activation in HUVECs that were pretreated with TAT-CaMBD or TAT (negative control) upon thrombin stimulation was determined using a Ral-GTP–specific pulldown. HUVECs were preincubated for 30 minutes in SF medium in the presence or absence of 200 μg/mL TAT-CaMBD or TAT peptide. Subsequently, cells were stimulated with 1 U/mL thrombin (T2) or SF-medium (−) for 2 minutes in the presence or absence of 200 μg/mL TAT-CaMBD or TAT peptide. The bottom panel shows total RalA as a loading control. (C) HUVECs were preincubated for 30 minutes with SF medium, 200 μg/mL TAT, or 200 μg/mL TAT-CaMBD. Subsequently, 1 U/mL thrombin, 10 mM epinephrine, and 100 mM IBMX or SF medium (unstimulated) was added for 15 minutes. WPBs were visualized by immunofluorescent staining of VWF. The number of remaining WPBs per cell upon stimulation in absence or presence of TAT or TAT-CaMBD was quantified using confocal microscopy. ***P < .001 by Student t test.
A cell-permeable peptide comprising the N-terminal CaMBD of RalGDS inhibits thrombin-induced WPB exocytosis. (A) The biotin-labeled peptides TAT-CaMBD, TAT (negative control), and TAT-Ral-c (positive control) were coupled to streptavidin-sepharose and used in a pull-down experiment from HUVEC lysate as described in “CaM pull-down assays.” The bottom panel shows total CaM levels to show equal expression. (B) RalA activation in HUVECs that were pretreated with TAT-CaMBD or TAT (negative control) upon thrombin stimulation was determined using a Ral-GTP–specific pulldown. HUVECs were preincubated for 30 minutes in SF medium in the presence or absence of 200 μg/mL TAT-CaMBD or TAT peptide. Subsequently, cells were stimulated with 1 U/mL thrombin (T2) or SF-medium (−) for 2 minutes in the presence or absence of 200 μg/mL TAT-CaMBD or TAT peptide. The bottom panel shows total RalA as a loading control. (C) HUVECs were preincubated for 30 minutes with SF medium, 200 μg/mL TAT, or 200 μg/mL TAT-CaMBD. Subsequently, 1 U/mL thrombin, 10 mM epinephrine, and 100 mM IBMX or SF medium (unstimulated) was added for 15 minutes. WPBs were visualized by immunofluorescent staining of VWF. The number of remaining WPBs per cell upon stimulation in absence or presence of TAT or TAT-CaMBD was quantified using confocal microscopy. ***P < .001 by Student t test.
Next, we investigated whether the TAT-CaMBD peptide could also interfere with thrombin-induced Ral activation and WPB exocytosis. HUVECs were preincubated for 30 minutes with 200 μg/mL TAT as a peptide control, 200 μg/mL TAT-CaMBD, or serum-free (SF) medium alone. Subsequently, these cells were incubated for 2 minutes with SF medium or thrombin (1 U/mL), and a Ral activation assay was performed. Thrombin induced a marked increase in the amount of active Ral in untreated and TAT-treated cells. However, treatment with TAT-CaMBD greatly inhibited thrombin-induced Ral activation (Figure 5B). Consistently, TAT-CaMBD also effectively inhibited thrombin-induced WPB exocytosis, as the number of remaining WPBs after thrombin stimulation was significantly higher in the presence of TAT-CaMBD; as expected, TAT-CaMBD did not affect degranulation induced by the cAMP-mediated agonist epinephrine (Figure 5C). These results indicate that TAT-CaMBD effectively competes with endogenous RalGDS for CaM binding, thereby preventing thrombin-induced Ral activation and WPB exocytosis from endothelial cells.
Discussion
Ral is involved in exocytotic processes through its binding and regulation of the exocyst complex, required for targeting secretory vesicles to specific sites on the plasma membrane,16 and through activation of PLD, which promotes the fusion of plasma and vesicle membranes.19 We have previously shown that RalA is involved in agonist-induced release of WPB in endothelial cells.13,14 In this study, we provide evidence that the Ral-specific GEF, RalGDS, is involved in exocytosis of WPBs. To our knowledge, this is the first identification of a RalGEF responsible for Ral-dependent exocytosis of dense core secretory granules.
RalGDS is best known for its function in the Ras-RalGDS-Ral pathway, leading to potentiation of cellular transformation induced by oncogenic Ras.30,31 Stimuli that are known to activate Ras, such as epidermal growth factor (EGF) or insulin, also lead to the activation of Ral, in a Ras-dependent manner.32 Activation of this pathway is achieved by association of the C-terminal Ras-binding domain (RBD) of RalGDS with membrane-tethered active Ras, which results in the translocation of RalGDS to the membrane and subsequent activation of its membrane-associated substrate Ral.33-35 Deletion of the RBD region prevents RalGDS recruitment to the membrane and also impairs its ability to activate Ral.35 Consistent with these findings, we observed that a RalGDS variant with its 145 carboxyterminal residues removed (thus also lacking its RBD) is not targeted to the plasma membrane, does not activate Ral, and does not lead to exocytosis of WPBs (data not shown). This observation suggests that targeting of RalGDS to the plasma membrane is essential for Ral-mediated exocytosis of WPBs.
Previously, the activity of RalGDS was found to be regulated by GPCR signaling via receptor activation-mediated dissociation of cytosolic, RalGDS/β-arrestin complexes.20 β-Arrestins are involved in desensitization and internalization of GPCRs,36 but they also regulate recruitment, activation, and scaffolding of receptor-induced signaling complexes.37 Reports that β-arrestins are linked to both protease activated receptor and β2-adrenergic receptor signaling38-41 are consistent with a role for RalGDS/β-arrestin complexes in the regulation of exocytosis of WPBs.
The findings reported in the current study indicate that RalGDS interacts with CaM via an IQ-motif that is present in the amino-terminal part of RalGDS. Similarly, Ras-GRF1, CDC25Mm, and Ras-GRF2, guanine exchange factors for Ras, contain IQ-motifs that enable their interaction with CaM.42-45 Different effects on GDP/GTP exchange have been observed after binding of these GEFs to CaM via their IQ-motifs. Activation of the guanine exchange factor Ras-GRF is mediated by calmodulin (CaM) binding to an IQ-motif in the amino terminal part of the Ras-GRF protein.43,45 However, CaM binding to Ras-GRF2 did not enhance its activity,44 and binding of CaM to CDC25Mm resulted in partial inhibition of the activity of this GEF.42 The amino-terminal domains of Ras-GRF, Ras-GRF2, and CDC25Mm restrain the actual exchange activity, because (partial) deletion of the N-terminal domains resulted in increased catalytic activity.42-44 It appears that the amino-terminal domains act as an autoinhibitory moiety; GDP/GTP exchange by the CDC25 domain of these GEFs is fully developed only when engaged by CaM and/or additional signaling factors. The amino terminal domain of RalGDS is also involved in regulating its GEF activity.46,47 The present study suggests that the interaction of CaM with the IQ-motif in the N-terminal domain of RalGDS positively contributes to its exchange activity. We cannot fully exclude that depletion of CaM using the TAT-CaMBD peptide also interferes with interactions with other (yet unknown) factors that may contribute to Ral activation and/or WPB exocytosis, however this is less likely because the TAT-CaMBD peptide did not interfere with WPB exocytosis induced by cAMP-dependent agonists such as epinephrine (Figure 5C). Several observations are consistent with the concept that CaM enhances activation of Ral. Studies by Birch et al have shown that inhibition of CaM reduces thrombin-induced release of VWF from WPBs in minimally permeabilized endothelial cells.48 In HUVECs treated with the CaM inhibitor trifluorperazine (TFP), thrombin-induced Ral activation was delayed and VWF secretion was significantly reduced.13 Wang et al have shown that Ral directly interacts with CaM,49 and, surprisingly, this interaction was shown to enhance GTP binding to Ral by 2- to 3-fold.26 The molecular mechanism underlying this effect remains to be clarified, but it was suggested that CaM induces a conformational change that promotes GTP binding to Ral. Our data indicate that CaM plays an important role in Ral activation by binding to (and possibly activating) RalGDS. The role of CaM-mediated activation of RalGDS is underscored by the fact that down-regulation of RalGDS impairs activation of Ral in vivo, which indicates that the interaction of CaM with Ral does not suffice for the rapid activation of Ral following stimulation of endothelial cells by thrombin. We propose that binding of Ca2+/CaM to the exchange factor RalGDS relieves the autoinhibitory property of the N-terminal domain and thereby enhances GTP-GDP exchange. Simultaneous binding of Ral and RalGDS to CaM may further enhance the efficiency of GTP-GDP exchange by RalGDS. Calmodulin also promotes thrombin-induced activation of Ral in human platelets,50 which raises the possibility that RalGDS is also involved in thrombin-induced Ral activation in platelets.
Exocytosis of WPBs induced by cAMP-mediated agonists, such as epinephrine, also involves the activation of Ral. The cAMP-activated protein kinase A (PKA) acts upstream of Ral activation as inhibition of PKA resulted in impeded VWF secretion and a total abrogation of Ral activation in response to epinephrine.14 The present study implicates RalGDS in cAMP-mediated degranulation because down-regulation of RalGDS expression or expression of a dominant negative RalGDS variant led to a decrease in epinephrine-induced WPB release. The question remains how the cAMP-dependent kinase PKA is able to confer its activation upon RalGDS. A number of guanine exchange factors have been described whose activity is regulated through phosphorylation by protein kinases, positively51-55 as well as negatively.56 RalGDS harbors a number of PKA phosphorylation recognition sites, and it can indeed be phosphorylated in vitro by PKA.57 However, phosphorylation of RalGDS did not significantly influence the in vitro GDP/GTP-exchange activity toward its substrate.23,57 Alternatively, phosphorylation of RalGDS by PKA may contribute to Ral activation by regulating the localization of RalGDS rather than its activity. Stimulation of the cAMP signaling pathway of human mesangial cells by endothelin 1 (ET-1) or forskolin resulted in the GTP loading of Cdc42 by inducing the translocation of the Rho family guanine exchange factor Pix. Inhibition of PKA blocked ET-1–induced Pix phosphorylation, which controls Pix translocation and consequently Cdc42 activation.58 Future studies should reveal the precise mechanism by which PKA regulates RalGDS-mediated release of WPBs.
In conclusion, our study supports the hypothesis that thrombin induces WPB exocytosis through the activation of Ral by the Ral-specific GEF RalGDS, after activation of RalGDS by Ca2+/CaM. It also shows that RalGDS is responsible for Ral-dependent release of WPBs after activation of a cAMP-mediated pathway, which we speculate involves translocation and/or activation of RalGDS following phosphorylation by PKA. Based on our findings, we propose the following model for RalGDS-mediated exocytosis of WPBs (Figure 6). The rise in intracellular levels of second messenger Ca2+ triggered by the stimulation of endothelial cells with thrombin leads to an association of free Ca2+ with the calcium sensor CaM. The Ca2+/CaM complex interacts with the calmodulin-binding domain situated in the amino-terminal region of RalGDS. Through this interaction, Ca2+/CaM sequesters the autoinhibitory amino-terminal region of RalGDS, thereby enhancing the exchange activity of the catalytic CDC25 domain of RalGDS. Simultaneously, the activation of an upstream GTPase that can interact with the carboxy-terminal RBD region of RalGDS leads to the translocation of the exchange factor to the plasma membrane where activation of Ral is needed to coordinate the exocyst complex and to promote PLD-induced fusion of vesicular and plasma membrane resulting in exocytosis of secretory granules.
Proposed mechanism for the regulation of Ca2+- and cAMP-mediated WPB exocytosis by RalGDS. (1) In unstimulated endothelial cells, RalGDS is complexed with β-arrestin in the cytoplasm, with the N-terminal part of RalGDS shielding off its catalytic GEF domain. (2) Thrombin stimulation leads to the release of Ca2+ in a PLC-dependent manner59 and to the dissociation of the RalGDS-β-arrestin complex. (3) Ca2+/CaM complex interacts with the IQ-motif present in the N-terminus of RalGDS, inducing a conformational change that relieves RalGDS of autoinhibition of its exchange activity. (4) Simultaneously, RalGDS translocates to the plasma membrane in a RBD-dependent manner by association of its RBD domain with a membrane-tethered active GTPase. (5) Membrane-associated active RalGDS activates Ral, which mediates release of WPBs through the coordination of the exocyst complex and PLD-induced fusion of vesicular and plasma membrane. (6) Triggering of the β2-adrenergic receptor leads to a rise in intracellular cAMP through the action of adenylate cyclase (AC). (7) cAMP-activated protein kinase A phosphorylates RalGDS, causing its translocation and/or partial activation, eventually leading to Ral activation and WPB exocytosis.
Proposed mechanism for the regulation of Ca2+- and cAMP-mediated WPB exocytosis by RalGDS. (1) In unstimulated endothelial cells, RalGDS is complexed with β-arrestin in the cytoplasm, with the N-terminal part of RalGDS shielding off its catalytic GEF domain. (2) Thrombin stimulation leads to the release of Ca2+ in a PLC-dependent manner59 and to the dissociation of the RalGDS-β-arrestin complex. (3) Ca2+/CaM complex interacts with the IQ-motif present in the N-terminus of RalGDS, inducing a conformational change that relieves RalGDS of autoinhibition of its exchange activity. (4) Simultaneously, RalGDS translocates to the plasma membrane in a RBD-dependent manner by association of its RBD domain with a membrane-tethered active GTPase. (5) Membrane-associated active RalGDS activates Ral, which mediates release of WPBs through the coordination of the exocyst complex and PLD-induced fusion of vesicular and plasma membrane. (6) Triggering of the β2-adrenergic receptor leads to a rise in intracellular cAMP through the action of adenylate cyclase (AC). (7) cAMP-activated protein kinase A phosphorylates RalGDS, causing its translocation and/or partial activation, eventually leading to Ral activation and WPB exocytosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from The Netherlands Heart Foundation (The Hague, The Netherlands; grants 2000.097 and 2002B187) and a grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR, Amsterdam, The Netherlands; 03.15). R.B. is an MRC (London, United Kingdom) Career Development Fellow. M.J.H. is an MRC Career-Track Scientist.
Authorship
Contribution: M.G.R., R.B., E.L.v.A., K.A.G., E.S., and A.K. performed experiments; M.G.R. and R.B. analyzed the data; S.S.G.F. and M.J.H. provided vital reagents, expertise, and protocols; K.M., J.A.v.M., M.F.-B., and J.V designed the study; M.G.R., R.B., J.A.v.M., M.F.-B., and J.V. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Voorberg, Department of Plasma Proteins, Sanquin Research and Landsteiner Laboratory, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: j.voorberg@sanquin.nl.
References
Author notes
*M.G.R. and R.B. contributed equally to this work.