Abstract
A fundamental property of platelets is their ability to transmit cytoskeletal contractile forces to extracellular matrices. While the importance of the platelet contractile mechanism in regulating fibrin clot retraction is well established, its role in regulating the primary hemostatic response, independent of blood coagulation, remains ill defined. Real-time analysis of platelet adhesion and aggregation on a collagen substrate revealed a prominent contractile phase during thrombus development, associated with a 30% to 40% reduction in thrombus volume. Thrombus contraction developed independent of thrombin and fibrin and resulted in the tight packing of aggregated platelets. Inhibition of the platelet contractile mechanism, with the myosin IIA inhibitor blebbistatin or through Rho kinase antagonism, markedly inhibited thrombus contraction, preventing the tight packing of aggregated platelets and undermining thrombus stability in vitro. Using a new intravital hemostatic model, we demonstrate that the platelet contractile mechanism is critical for maintaining the integrity of the primary hemostatic plug, independent of thrombin and fibrin generation. These studies demonstrate an important role for the platelet contractile mechanism in regulating primary hemostasis and thrombus growth. Furthermore, they provide new insight into the underlying bleeding diathesis associated with platelet contractility defects.
Introduction
The ability of platelets to adhere to subendothelial matrix proteins and with other platelets at sites of vascular injury is critical for hemostatic plug formation and for the development of arterial thrombi.1,2 The hemostatic response can be divided into 2 temporally distinct phases: the primary hemostatic plug, composed of aggregated platelets that form independent of fibrin formation1 ; and the secondary hemostatic response, wherein fibrin polymers enmeshed into the developing thrombus physically stabilize the platelet plug.3 During hemostatic plug formation, platelets undergo a complex series of morphologic and functional responses that require extensive remodeling of the actin cytoskeleton. These cytoskeletal changes are indispensable for the normal hemostatic function of platelets and are controlled by complex network of signaling, structural, and regulatory proteins.4
The actin-based cytoskeleton can be separated into 2 functionally distinct structures: (1) the spectrin-rich membrane skeleton, lining the inner plasma membrane, and (2) the cytoskeleton, consisting of long actin filaments that radiate from the cell center to the surface membrane.4,5 The membrane skeleton is essential for maintaining the structure and integrity of the surface membrane, whereas the cytoskeleton, through its attachment to myosin, principally generates contractile forces within the cell.6 The internal generation of contractile force has a well-defined role in regulating platelet shape change7,8 and in promoting granule secretion,9,10 whereas the extracellular transmission of cytoskeletal contractile force is essential for fibrin clot retraction.11,12
Central to the generation of contractile force is the molecular motor myosin, representing one of the major proteins found in the platelet cytosol.4,6,13 Platelets exclusively express the nonmuscle myosin IIA,14 with deficiency of this isoform leading to the complete loss of platelet contractile function.15 Platelet contractility requires phosphorylation of myosin light chains (MLCs), which is under the dual control of myosin light chain kinase (MLCK) and myosin phosphatase (mPP).4,16,17 Both MLCK and mPP are regulated by calcium/calmodulin and Rho kinase, respectively, and in most cell types calcium and Rho kinase contribute to the development of contractility. In platelets, calcium appears to be the predominant regulator of contractile force generation, as inhibition of Rho kinase has minimal effect on fibrin clot retraction18 and only inhibits platelet shape change under experimental conditions limiting cytosolic calcium flux.19-21 Nonetheless, Rho kinase appears to play an important role in platelet function, by promoting focal adhesion–like complexes18 and actin stress fibers in spreading platelets.22 Such structures may play a key role in regulating the adhesive function of platelets, as inhibition of Rho kinase undermines the stability of platelet-matrix and platelet-platelet interactions in a shear field,23 leading to a major defect in thrombus growth.22
Genetic abnormalities in myosin IIA (termed the MYH9 disorders) encompass several autosomal dominant disorders, including the May-Hegglin anomaly and Epstein, Sebastian, and Fechtner syndromes.24,25 A hallmark feature of these disorders is the failure of platelets to undergo shape change following soluble agonist stimulation, whereas platelet aggregation remains intact. Most of these patients exhibit minor bleeding disorders, although in a small subset of patients bleeding can be severe.26 Recent studies on mice with a targeted deletion of myosin IIA in platelets have confirmed the importance of the platelet contractile mechanism in supporting the hemostatic function of platelets, leading to a major prolongation in tail bleeding time and a severe defect in thrombus growth.15 Complete deficiency of myosin IIA abolished platelet shape change and clot retraction; however, platelet aggregation and granule release largely remains intact.15
While the importance of the platelet contractile mechanism in supporting the hemostatic function of platelets is well defined, it currently remains unclear how important contractility is to the regulation of the primary hemostatic plug, independent of blood coagulation and fibrin clot retraction. In the current study, we have investigated the role of the platelet contractile apparatus in regulating the dynamics of platelet aggregate formation under physiologic blood flow conditions. By performing real-time analysis of platelet adhesion and aggregation on a collagen substrate we have demonstrated a distinct contractile phase to thrombus development that occurred independent of thrombin generation and fibrin polymerization. We demonstrate that in contrast to fibrin clot retraction, platelet thrombus contraction was primarily mediated by signaling events linked to Rho kinase, with inhibition of contractility undermining the tight packing of aggregated platelets, leading to instability of the primary hemostatic plug. These studies demonstrate the existence of a previously unrecognized Rho kinase–dependent contractile mechanism regulating primary hemostasis and thrombus growth. These new findings may help explain the bleeding tendency of individuals with defects in platelet contractility.
Methods
Materials
The Rho kinase inhibitor H1152 was obtained from Toronto Research Chemicals (North York, ON). IP3 receptor antagonist 2-aminoethoxydiphenyl borate (2-APB) was from Cayman Chemicals (Ann Arbor, MI). HA1077, the myosin II inhibitor blebbistatin, and its inactive enantiomer were obtained from Chemicon (Temecula, CA). DiIC12 was from BD Biosciences (Rockville, MD). Recombinant hirudin (lepirudin) was purchased from Celgene (Summit, NJ). All other reagents were from sources previously described.27-29
Mouse strains
All procedures involving the use of C57Bl6 and PAR4−/− mice were approved by the Alfred Medical Research and Education Precinct (AMREP) animal ethics committee (AEC, Melbourne, Australia), under project numbers E/0569/2007/M, E/0621/2007/M, and E/0464/2006/M.
Collection of blood, preparation of PRP and washed platelets
All procedures involving collection of human and mouse blood were approved by the Monash University Standing Committee on Ethics in Research involving Humans (SCERH) (project number CF07/0125-2007/0005) and the AMREP AEC (SOP19, collection of whole blood from mice), respectively. For isolation of human platelet-rich plasma (PRP), whole blood from consenting healthy volunteers was collected into trisodium citrate (0.38% final concentration), and centrifuged at 300g for 16 minutes at 37°C. Washed platelets were prepared from acid-citrate dextrose (ACD)–anticoagulated whole blood, according to the method of Schoenwaelder et al,30 with the inclusion of lepirudin (800 U/mL) in the platelet washing buffer and apyrase (0.02 U/mL) in the Tyrode buffer.
Visualization and quantification of in vitro thrombus consolidation under flow
Flow-based thrombus formation assays on a bovine fibrillar type I collagen matrix were performed at 37°C in the absence of fibrin as described previously.29 Briefly, anticoagulated (800 U/mL lepirudin) human whole blood was preincubated with vehicle (DMSO), blebbistatin (200 μM), an inactive enantiomer of blebbistatin (control, 200 μM), EGTA/Mg2+ (2 mM/1 mM), HA1077 (80 μM), H1152 (40 μM), or 2-APB (200 μM; 10 minutes, 37°C) prior to perfusion through fibrillar type I collagen–coated microcapillary tubes (2.0 mg/mL) at 1800 s−1 for 5 minutes. Thrombus formation was observed using an inverted Leica DMIRB microscope (Leica Microsystems, Wetzlar, Germany) with a 63× water objective (1.2 numeric aperture [NA]), and recorded in real time using a Dage-MTI charge-coupled device (CCD) camera 300 ETRCX (Dage-MTI, Michigan City, IN).
Two-dimensional quantification of thrombus consolidation.
Quantification of thrombus contraction was performed by “spiking” whole blood with 3% DiIC12-labeled platelets prior to perfusion. Spiked whole blood was then perfused over collagen matrices as described, and DIC/fluorescence images were recorded in real-time as described above, for off-line analysis. Studies examining the effect of 2-APB on consolidation were performed by perfusing untreated whole blood across microslides for 30 seconds to establish a nucleating thrombus, followed by perfusion of inhibitor treated blood. This preinhibitor perfusion was necessary as the presence of 2-APB prevents thrombus formation, precluding the analysis of consolidation. The distance between 2 fluorescently labeled platelets in a given thrombus was measured every 30 seconds over 5 minutes. Results are expressed as the decrease in the distance between 2 platelets (interplatelet distance) over time, relative to interplatelet distance measured at 1′ (taken as 100%).
Three-demensional volumetric thrombus analysis.
For analysis of thrombus volume, whole blood was labeled with DiIC12 (1 μM) prior to perfusion. Thrombi were formed as described, and images captured in real time using an inverted Leica DMIRB confocal microscope, with 1-μM sections acquired every 30 seconds over 4 to 5 minutes. Analysis of thrombus volume was performed using Metamorph 6 software.
Intravital microscopy
The development and consolidation of thrombi in response to vessel injury was monitored using intravital microscopy. C57BL6 or PAR4 deficient (15-18 g) mice were anesthetized using sodium pentobarbitone (60 mg/kg), and the mesentery exteriorized through a midline abdominal incision. Body temperature was maintained during the procedure using an infrared heat lamp, and exposed mesenteric vessels (50 μm-160 μm diameter) were hydrated using warm saline. Vessel injury was achieved either through vessel puncture using a microinjection needle (20- to 30-μm tip diameter) connected to a micromanipulator (Eppendorf, Hamburg, Germany), or through application of 6% FeCl3-soaked filter paper (8 seconds). Accrual of platelets to the area of injury was recorded in real time as described for in vitro flow assays. In some experiments, H1152 (5 mM stock solution, 2.5 μL injection volume per cycle), HA1077 (10 mM stock solution, 2.5 μL injection volume per cycle), 2-APB (25 mM stock solution, 2.5 μL injection volume per cycle), blebbistatin or its inactive enantiomer (25 mM stock solution, 2.5 μL total injection volume), or an equivalent volume of vehicle (DMSO), were locally infused into developing thrombi via the microinjection needle (release rate 2-3 μL/min, 3 cycles). To prevent fibrin generation, in some experiments, lepirudin (50 mg/kg) was administered via intravenous injection prior to induction of injury and subsequent injection of inhibitors. Complete abolition of fibrin formation at this concentration of lepirudin was confirmed by histology using Carstair stain.30 The surface area of thrombi in vivo was measured using Image J, with analysis of every fifth frame (at 1 frame/sec) over 4 to 5 minutes. Change in surface area was expressed as fold-increase or decrease over the original surface area (given an arbitrary value of 1.0).
Platelet-mediated fibrin clot retraction
Statistical analysis
Statistical significance between multiple treatment groups was analyzed using a 1-way analysis of variance (ANOVA) with Dunnett multiple comparison test. Statistical significance between multiple treatment groups over time was performed using 2-way ANOVA, with Bonferroni post-tests. Statistical significance between 2 treatment groups was analyzed using an unpaired Student t test with 2-tailed P values (Prism software; GraphPAD Software for Science, San Diego, CA). Data are presented as means plus or minus either the standard error of the mean (SEM) or standard deviation (SD; where indicated), where n equals the number of independent experiments performed.
Results
Identification of a contractile phase during thrombus development
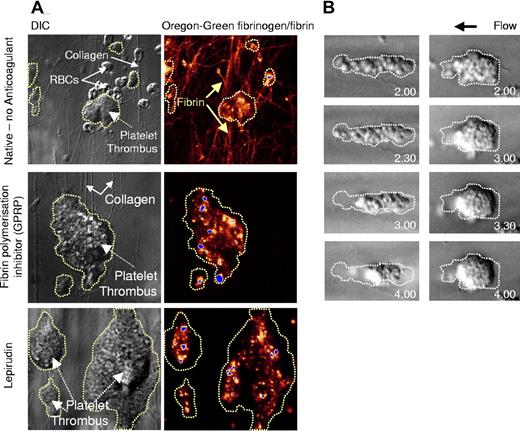
The importance of platelets in transmitting cytoskeletal contractile forces to fibrin polymers, leading to clot retraction, is well defined.11,12,15,33 However, the importance of these contractile mechanisms in regulating the various stages of thrombus growth, particularly under physiologic blood flow conditions, has been less clearly defined. To investigate this, we used an in vitro perfusion system that allows real-time analysis of platelet thrombus growth on an immobilized type I fibrillar collagen substrate. In this report, the term “platelet thrombus” refers to the cumulative effects of both primary adhesion and platelet aggregation under flow conditions, irrespective of blood coagulation. Perfusion of native (nonanticoagulated) whole blood at arteriolar shear rates (1800 s−1) resulted in rapid platelet adhesion and aggregate formation, with the formation of large stable aggregates within 2 minutes of flow (Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Analysis of deposited fibrin(ogen), by coperfusing fluorescently labeled fibrinogen, revealed widespread fibrin(ogen) incorporation within the developing thrombus, with individual thick fibrin strands prominent around the base of thrombi and over the collagen surface (Figure 1A). Concomitant with thrombus formation, a time-dependent contraction of thrombi was observed (Video S1). Contraction of thrombi was apparent within the first 60 seconds of flow and was continuous throughout the 5-minute perfusion period. High-resolution imaging of thrombi revealed that thrombus contraction was associated with the progressive tight packing of aggregated platelets, such that the margins of individual platelets could no longer be distinguished within the developing thrombus. Notably, retraction of platelets into the developing thrombus occurred prior to the development of thick fibrin polymers (Video S1), raising the possibility that this process occurred independent of fibrin polymerization. To investigate this, we performed perfusion studies in the presence of the Gly-Pro-Arg-Pro peptide (GPRP), an inhibitor of fibrin polymerization. The addition of GPRP to native whole blood inhibited the formation of individual fibrin polymers but had no inhibitory effect on platelet thrombus growth (Figure 1A). Furthermore, the contraction of platelet thrombi was unaltered by GPRP (data not shown). Similarly, thrombi formed using hirudin-anticoagulated whole blood also underwent a prominent contractile phase, leading to marked consolidation of forming thrombi (Video S2, Figure 1B). To exclude the possibility that trace amounts of thrombin were responsible for this contractile process, we performed studies on mouse platelets that are completely unresponsive to thrombin stimulation (PAR4−/− mice). As demonstrated in Video S2, PAR4 deficiency had no inhibitory effect on thrombus contraction or on the consolidation of forming thrombi. Moreover, treating whole blood with very high concentrations of lepirudin (1600 U/mL), in combination with the low molecular weight heparin, enoxaparine (400 U/mL), also did not prevent thrombus contraction (data not shown), confirming that this phenomenon occurred independent of thrombin generation and fibrin polymerization.
Fibrin-independent thrombus contraction. Human whole blood was collected in the absence of anticoagulant (Native) (A), in the presence of the fibrin polymerization inhibitor GPRP (GPRP, 280 μM) (A), or in the presence of lepirudin (800 U/mL) (A,B), then perfused through type I collagen–coated glass microslides at 1800 s−1 for up to 5 minutes. (A) To visualize fibrin formation, whole-blood perfusion was performed in the presence of Oregon green–labeled fibrinogen (20 μg/mL). DIC and fluorescence images were captured in real-time using a Leica inverted microscope (×63 magnification). Images are taken from 1 representative of 4 independent experiments. (B) Thrombus formation and consolidation during perfusion of lepirudin-anticoagulated whole-blood was recorded in real time using DIC microscopy, and snapshots of individual thrombi at the indicated time points were obtained off-line. These images are taken from 1 representative of 12 independent experiments. The original outline of the thrombus prior to contraction is highlighted by the broken line.
Fibrin-independent thrombus contraction. Human whole blood was collected in the absence of anticoagulant (Native) (A), in the presence of the fibrin polymerization inhibitor GPRP (GPRP, 280 μM) (A), or in the presence of lepirudin (800 U/mL) (A,B), then perfused through type I collagen–coated glass microslides at 1800 s−1 for up to 5 minutes. (A) To visualize fibrin formation, whole-blood perfusion was performed in the presence of Oregon green–labeled fibrinogen (20 μg/mL). DIC and fluorescence images were captured in real-time using a Leica inverted microscope (×63 magnification). Images are taken from 1 representative of 4 independent experiments. (B) Thrombus formation and consolidation during perfusion of lepirudin-anticoagulated whole-blood was recorded in real time using DIC microscopy, and snapshots of individual thrombi at the indicated time points were obtained off-line. These images are taken from 1 representative of 12 independent experiments. The original outline of the thrombus prior to contraction is highlighted by the broken line.
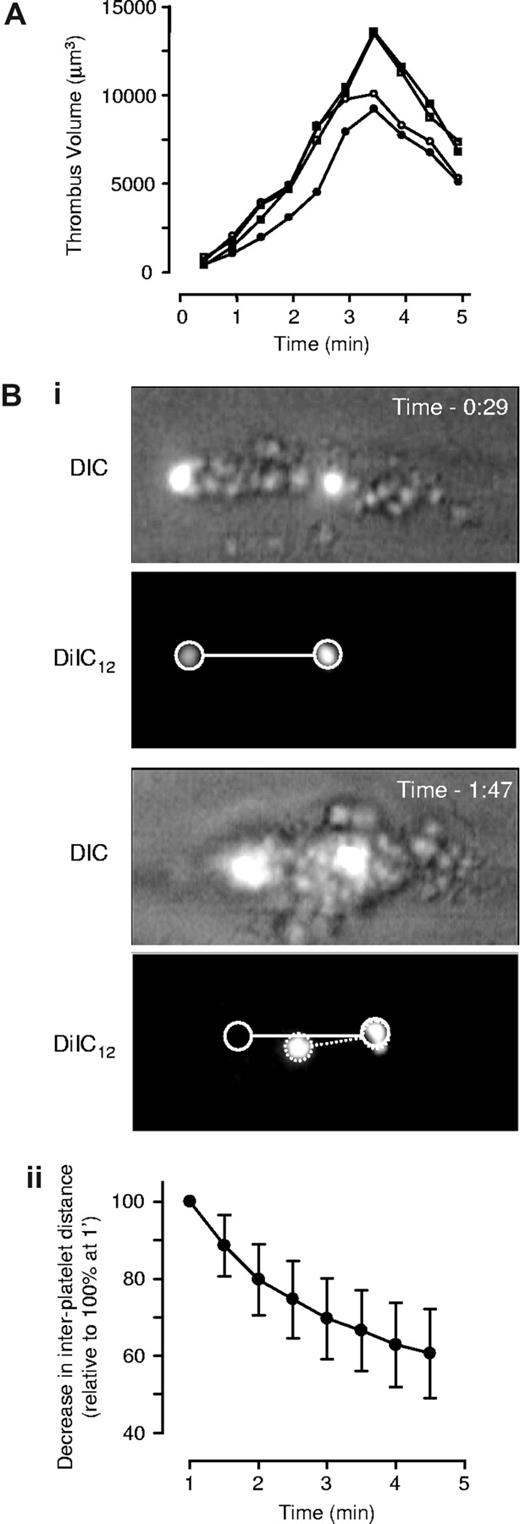
To determine the impact of contraction on the volume of forming thrombi, 3-D volumetric analysis of thrombi was performed. Hirudin-treated whole blood preincubated with the fluorescent membrane dye DiIC12 was perfused over type I collagen at 1800 s−1, and confocal sections of developing thrombi taken at 30-second intervals over a 5-minute time period. Thrombi were reconstructed in 3 dimensions and volume quantified as described in “Three-dimensional volumetric thrombus analysis.” As demonstrated in Figure 2A, the volume of individual thrombi increased in a time-dependent manner (volume of individual thrombi ranging from ∼5000 μm3 up to 15 000 μm3), with maximal thrombus size apparent after 3 to 3.5 minutes of flow. Contraction occurred continuously throughout thrombus development; however, it was not until after 3.5 minutes of flow that a net decrease in thrombus volume was apparent, with an overall decrease between 23.9% to 48.2% (mean 38.2% ± 16.1% SD, n = 10). Thrombus contraction typically involved the retraction of individual platelets into the body of the developing thrombus with the most rapid contraction occurring in the downstream tail of the developing thrombus (Figure 1B). To quantify the change in distance between individual platelets during thrombus contraction, we established a fluorescence-based tracking method that enabled analysis of movement of individual platelets following stable incorporation into thrombi (Figure 2Bi; see “Two-dimensional quantification of thrombus consolidation”). These studies revealed a time-dependent reduction in the distance between individual platelets, ranging from 12.5% to 62.5% (mean 37.7% ± 12.8% SD, n = 36; Figure 2Bii).
Characterization of thrombus consolidation in vitro. Lepirudin-anticoagulated human whole-blood was perfused through collagen-coated microslides at 1800 s−1. (A) To quantify thrombus volume, whole blood was preincubated with DiIC12 prior to perfusion, and three-dimensional images captured in real-time using an inverted Leica DMIRB confocal microscope, followed by off-line analysis to quantify thrombus volume, as described in “Three-dimensional volumetric thrombus analysis.” This graph depicts thrombus volume over time from 4 individual thrombi taken from 4 independent experiments. (B) Quantification of thrombus consolidation was performed by “spiking” whole blood with DiIC12-labeled platelets prior to perfusion, followed by capture of consecutive DIC and fluorescence images in real-time. (i) Images are taken from 1 flow representative of 12 independent flows. (ii) The decrease in distance between firmly adherent platelets was quantified as described in “Two-dimensional quantification of thrombus consolidation” and used as an indirect marker of thrombus contraction. Results are expressed as the mean plus or minus SD of 36 individual thrombi, from 12 independent experiments (n = 12; —).
Characterization of thrombus consolidation in vitro. Lepirudin-anticoagulated human whole-blood was perfused through collagen-coated microslides at 1800 s−1. (A) To quantify thrombus volume, whole blood was preincubated with DiIC12 prior to perfusion, and three-dimensional images captured in real-time using an inverted Leica DMIRB confocal microscope, followed by off-line analysis to quantify thrombus volume, as described in “Three-dimensional volumetric thrombus analysis.” This graph depicts thrombus volume over time from 4 individual thrombi taken from 4 independent experiments. (B) Quantification of thrombus consolidation was performed by “spiking” whole blood with DiIC12-labeled platelets prior to perfusion, followed by capture of consecutive DIC and fluorescence images in real-time. (i) Images are taken from 1 flow representative of 12 independent flows. (ii) The decrease in distance between firmly adherent platelets was quantified as described in “Two-dimensional quantification of thrombus consolidation” and used as an indirect marker of thrombus contraction. Results are expressed as the mean plus or minus SD of 36 individual thrombi, from 12 independent experiments (n = 12; —).
Importance of Rho kinase for thrombus contraction
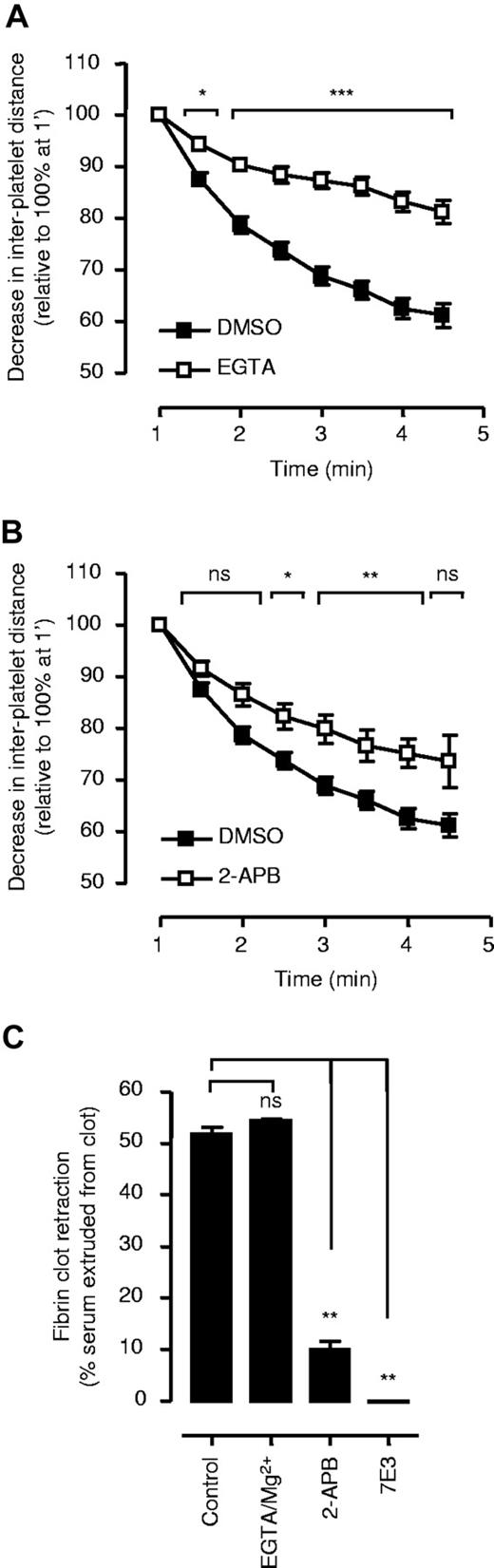
Actinomyosin-based contractility is tightly linked to the phosphorylation of MLCK, through calcium/calmodulin-dependent activation of MLCK and Rho kinase–dependent inactivation of myosin phosphatase.16,17 In platelets, calcium-activation of MLCK appears to be the dominant contractile mechanism regulating platelet shape change19-21 and fibrin clot retraction.18 To investigate the role of cytosolic calcium flux in regulating thrombus contraction, whole-blood perfusion studies were performed under experimental conditions preventing calcium influx (EGTA/MgCl2) or calcium mobilization from internal stores (IP3 receptor antagonist, 2-APB). Chelating extracellular calcium with EGTA significantly reduced the rate of thrombus contraction (up to 52% at 5 minutes perfusion P < .001, Figure 3A). Under similar assay conditions, inhibition of calcium mobilization (2-APB) had a less pronounced effect on thrombus contraction, reducing contraction by 32% (Figure 3B). This contrasted markedly with fibrin clot retraction, in which EGTA/MgCl2 had no significant inhibitory effect (Figure 3C) whereas APB abolished clot retraction at all time points examined (Figure 3C).
Role of calcium in regulating thrombus contraction. Lepirudin-anticoagulated human whole blood, spiked with DiIC12-labeled platelets, was perfused through collagen-coated microslides at 1800 s−1. The decrease in distance between firmly adherent platelets was quantified as described in “Two-dimensional quantification of thrombus consolidation” and used as an indirect marker of thrombus contraction. (A) Whole blood was perfused in the presence of EGTA/Mg2+ (2 mM/1 mM). (B) For studies with 2-APB, whole blood was initially perfused for 30 seconds without inhibitor to allow for the initial formation of noncontracted thrombi (refer to “Two-dimensional quantification of thrombus consolidation”), followed by perfusion of whole blood in the presence of 2-APB (200 μM). (A,B) Results represent the mean plus or minus SEM (n = 5; *P < .05; **P < .005; ***P < .001). (C) To examine the importance of calcium flux for fibrin clot retraction, PRP was preincubated with 2-APB (200 μM), c7E3 (50 μg/mL), or EGTA/Mg2+ (2 mM/1 mM), followed by addition of thrombin (1 U/mL). Clot retraction was assessed as described under “Platelet-mediated fibrin clot retraction.” Results represent the mean plus or minus SEM (n = 3; ns = P > .05; **P < .005).
Role of calcium in regulating thrombus contraction. Lepirudin-anticoagulated human whole blood, spiked with DiIC12-labeled platelets, was perfused through collagen-coated microslides at 1800 s−1. The decrease in distance between firmly adherent platelets was quantified as described in “Two-dimensional quantification of thrombus consolidation” and used as an indirect marker of thrombus contraction. (A) Whole blood was perfused in the presence of EGTA/Mg2+ (2 mM/1 mM). (B) For studies with 2-APB, whole blood was initially perfused for 30 seconds without inhibitor to allow for the initial formation of noncontracted thrombi (refer to “Two-dimensional quantification of thrombus consolidation”), followed by perfusion of whole blood in the presence of 2-APB (200 μM). (A,B) Results represent the mean plus or minus SEM (n = 5; *P < .05; **P < .005; ***P < .001). (C) To examine the importance of calcium flux for fibrin clot retraction, PRP was preincubated with 2-APB (200 μM), c7E3 (50 μg/mL), or EGTA/Mg2+ (2 mM/1 mM), followed by addition of thrombin (1 U/mL). Clot retraction was assessed as described under “Platelet-mediated fibrin clot retraction.” Results represent the mean plus or minus SEM (n = 3; ns = P > .05; **P < .005).
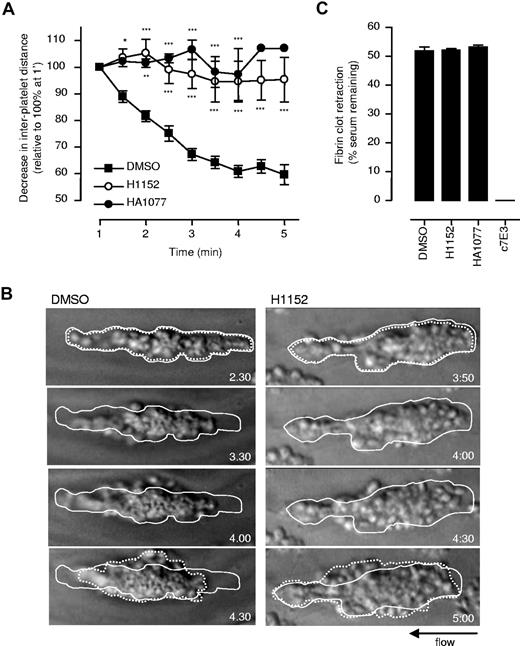
To examine the contribution of Rho kinase to thrombus contraction, the effects of the Rho kinase inhibitor H115234 were examined. H1152 had a marked effect on the thrombus contraction process, resulting in an 88% decrease after 5 minutes of perfusion (P < .001, Figure 4A,B). This defect in contraction was associated with reduced tight packing of platelets into the developing thrombus, leading to the formation of less stable thrombi (Figure 4B, Video S3). Similar findings were obtained with another Rho kinase inhibitor HA1077 (Figure 4A). These effects were selective to thrombus contraction, as neither inhibitor had any significant effect on the rate and extent of fibrin clot retraction (Figure 4C).
Role of Rho kinase in regulating thrombus contraction. (A) Lepirudin-anticoagulated human whole blood, spiked with DiIC12-labeled platelets, was preincubated with vehicle (DMSO), H1152 (40 μM), or HA1077 (80 μM), prior to perfusion through collagen-coated microslides at 1800 s−1. The interplatelet distance between firmly adherent platelets was quantified and used as an indirect marker of thrombus contraction. Results represent the mean plus or minus SEM (n = 4; *P < .05; **P < .005; ***P < .001). (B) Thrombus formation in the presence of vehicle (DMSO) or H1152 (40 μM) was recorded in real time, and snapshots of individual thrombi at the indicated times were taken off-line. The original size of the thrombus is outlined by a solid line, while the resultant thrombus size following 2 minutes of flow is outlined by a broken line. These images are taken from 1 representative of 4 independent experiments. (C) To examine the effect of Rho kinase inhibitors on fibrin-dependent clot retraction, citrated PRP was preincubated with vehicle (DMSO), H1152 (40 μM), HA1077 (80 μM), or c7E3 (100 μg/mL), followed by addition of thrombin (1.0 U/mL). The extent of clot retraction was assessed after 30 minutes, as described in “Platelet-mediated fibrin clot retraction.” Results represent the mean plus or minus SEM (n = 3).
Role of Rho kinase in regulating thrombus contraction. (A) Lepirudin-anticoagulated human whole blood, spiked with DiIC12-labeled platelets, was preincubated with vehicle (DMSO), H1152 (40 μM), or HA1077 (80 μM), prior to perfusion through collagen-coated microslides at 1800 s−1. The interplatelet distance between firmly adherent platelets was quantified and used as an indirect marker of thrombus contraction. Results represent the mean plus or minus SEM (n = 4; *P < .05; **P < .005; ***P < .001). (B) Thrombus formation in the presence of vehicle (DMSO) or H1152 (40 μM) was recorded in real time, and snapshots of individual thrombi at the indicated times were taken off-line. The original size of the thrombus is outlined by a solid line, while the resultant thrombus size following 2 minutes of flow is outlined by a broken line. These images are taken from 1 representative of 4 independent experiments. (C) To examine the effect of Rho kinase inhibitors on fibrin-dependent clot retraction, citrated PRP was preincubated with vehicle (DMSO), H1152 (40 μM), HA1077 (80 μM), or c7E3 (100 μg/mL), followed by addition of thrombin (1.0 U/mL). The extent of clot retraction was assessed after 30 minutes, as described in “Platelet-mediated fibrin clot retraction.” Results represent the mean plus or minus SEM (n = 3).
Inhibiting platelet contractility undermines the stability of platelet thrombi
The ability of the platelet contractile apparatus to promote tight packing of platelets within a developing thrombus suggests a potentially important role for contractility in maintaining thrombus stability. To investigate the importance of platelet contractility in this process we examined the effect of the myosin IIA inhibitor, blebbistatin, on thrombus growth and stability. As demonstrated in Figure 5 and Video S4, blebbistatin-treated platelets were able to adhere and form large aggregates on the type I fibrillar collagen substrate; however, the subsequent tight packing of platelets did not occur, leading to the development of highly unstable platelet thrombi. This lack of thrombus consolidation resulted in continual embolization of platelets from the thrombus surface, undermining the growth of forming thrombi (Figure 5, Video S4). To determine whether platelet contractility is important to maintain thrombus stability in vivo, we established an intravital thrombosis model in the mouse microcirculation that enables real-time dynamic analysis of thrombus growth and stability. In this model, platelet thrombi are induced in postcapillary venules by micropuncture of the vessel wall with a microinjector needle. Nonocclusive thrombi rapidly form at the site of injury and high magnification imaging revealed that thrombi formed under these conditions primarily consisted of platelets (Video S5). Consistent with this, pretreating mice with a platelet GPIb receptor antagonist (alboaggregin) or GPIIb-IIIa antagonist (GPI-162) completely eliminated thrombus formation (data not shown). High-magnification imaging of forming thrombi revealed the progressive tight packing of individual platelets within the core of the developing thrombus that was associated with thrombus contraction (Video S5). Similar findings were apparent with thrombi formed following FeCl3 induced vascular injury (data not shown), suggesting that thrombus contraction represented a general feature of thrombus growth in vivo. The local administration of blebbistatin into the microcirculation following thrombus formation resulted in the loss of tight packing between individual platelets, particularly in the outer layers of formed thrombi, leading to progressive embolization of platelet aggregates from the thrombus surface (Figure 6A) and a mean reduction in thrombus size by 38% (Figure 6B). In control studies, microinjection of vehicle alone or the inactive blebbistatin enantiomer had no effect (Figure 6A,B). Furthermore, with cessation of blebbistatin administration, thrombi rapidly reformed at the site of injury such that repetitive cycles of thrombus growth and embolization could be achieved with regular cycles of blebbistatin administration (Figure 6A).
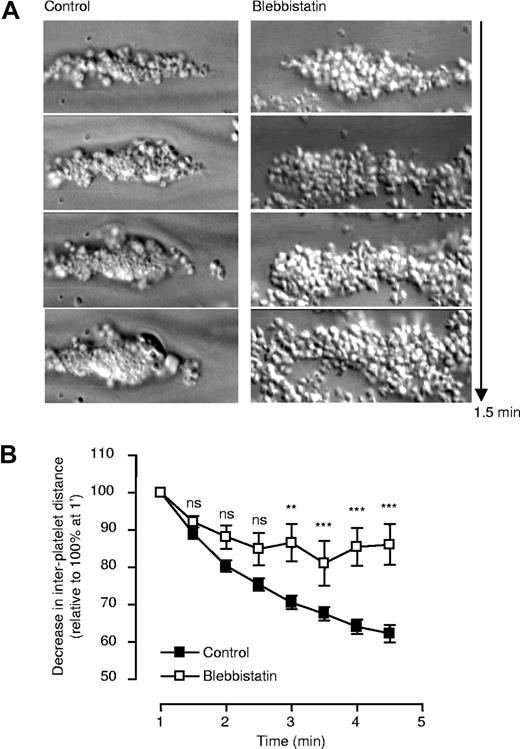
Effect of the myosin II inhibitor (blebbistatin) on thrombus consolidation in vitro. Lepirudin-anticoagulated human whole blood was preincubated with blebbistatin, or an inactive enantiomer of blebbistatin (control), prior to perfusion through collagen-coated microslides at 1800 s−1, as described in “Two-dimensional quantification of thrombus consolidation.” (A) Snapshots of individual thrombi over a time period of 1.5 minutes were obtained off-line. These images are taken from 1 representative of 3 independent experiments. (B) Whole blood spiked with DiIC12-labeled platelets was perfused through collagen-coated microslides at 1800 s−1, and the distance between firmly adherent platelets quantified, as described in “Two-dimensional quantification of thrombus consolidation.” Results represent the mean plus or minus SEM (n = 3; ns P > .05; **P < .005; ***P < .001).
Effect of the myosin II inhibitor (blebbistatin) on thrombus consolidation in vitro. Lepirudin-anticoagulated human whole blood was preincubated with blebbistatin, or an inactive enantiomer of blebbistatin (control), prior to perfusion through collagen-coated microslides at 1800 s−1, as described in “Two-dimensional quantification of thrombus consolidation.” (A) Snapshots of individual thrombi over a time period of 1.5 minutes were obtained off-line. These images are taken from 1 representative of 3 independent experiments. (B) Whole blood spiked with DiIC12-labeled platelets was perfused through collagen-coated microslides at 1800 s−1, and the distance between firmly adherent platelets quantified, as described in “Two-dimensional quantification of thrombus consolidation.” Results represent the mean plus or minus SEM (n = 3; ns P > .05; **P < .005; ***P < .001).
Role of myosin IIA and Rho kinase in regulating thrombus stability in vivo. Vascular injury was induced in the mesenteric postcapillary venules of anesthetized C57/Bl6 mice by needle puncture, and thrombus development recorded as described in “Intravital microscopy.” In the indicated experiments, the effects of blebbistatin, an inactive enantiomer of blebbistatin (control), vehicle (DMSO), or H1152 on thrombus stability was assessed following intermittent injections (denoted by ▀), with concentrations and volumes as described in “Intravital microscopy.” (A) The relative change in surface area of a given thrombus over time was determined off-line, and expressed relative to the initial surface area of the thrombus prior to injection. These results depict data taken from 1 of 4 independent experiments, with representative images from one such experiment depicted in panel B. The percentage decrease in thrombus surface area following injection was quantified as described in “Intravital microscopy,” and expressed as a percentage (%) of the original thrombus. These results represent the mean plus or minus SEM (n = 4), where *** indicates P < .001.
Role of myosin IIA and Rho kinase in regulating thrombus stability in vivo. Vascular injury was induced in the mesenteric postcapillary venules of anesthetized C57/Bl6 mice by needle puncture, and thrombus development recorded as described in “Intravital microscopy.” In the indicated experiments, the effects of blebbistatin, an inactive enantiomer of blebbistatin (control), vehicle (DMSO), or H1152 on thrombus stability was assessed following intermittent injections (denoted by ▀), with concentrations and volumes as described in “Intravital microscopy.” (A) The relative change in surface area of a given thrombus over time was determined off-line, and expressed relative to the initial surface area of the thrombus prior to injection. These results depict data taken from 1 of 4 independent experiments, with representative images from one such experiment depicted in panel B. The percentage decrease in thrombus surface area following injection was quantified as described in “Intravital microscopy,” and expressed as a percentage (%) of the original thrombus. These results represent the mean plus or minus SEM (n = 4), where *** indicates P < .001.
To investigate the role of Rho kinase in regulating thrombus stability, H1152 was locally administered into the microcirculation following thrombus development. Identical to the findings with blebbistatin, inhibiting Rho kinase undermined the sustained tight packing of aggregated platelets, particularly in the superficial layers of thrombi, leading to embolization of platelets from the thrombus surface (Video S6, Figure 6A) and a mean reduction in thrombus size by 34% (Figure 6B). In control studies, the local administration of vehicle (DMSO) control was without effect (Figure 6A,B). Rho kinase appeared to play the dominant role in this process, as H1152 was more effective than the IP3 receptor antagonist APB at inducing thrombus instability and embolization (data not shown). These studies define a major role for Rho kinase and the platelet contractile mechanism in maintaining thrombus stability in vivo.
Platelet contractility is essential for the stability of the primary hemostatic plug
To investigate whether thrombus contraction in vivo required thrombin stimulation of platelets, intravital microscopy studies were performed on PAR4−/− mice. The initial platelet adhesion and aggregation response of these platelets was normal following micropuncture of postcapillary venules; however, the thrombi that formed were less stable than PAR4+/+ controls, leading to repetitive cycles of thrombus formation and embolization, particularly in the superficial layers of forming thrombi. These findings confirm previous reports that thrombin-stimulation of platelets plays a critical role in stabilizing forming thrombi.35,36 Despite their instability, thrombi formed in PAR4−/− mice underwent a prominent contractile phase that led to thrombus consolidation, particularly in the core of forming thrombi (Video S5).
To eliminate thrombin, and thereby exclude the possible involvement of fibrin clot retraction to this process, wild-type mice were pretreated with high-dose lepirudin (50 mg/kg) prior to vessel injury. Mural thrombus formation occurred rapidly following needle puncture of postcapillary venules; however, in the absence of thrombin, thrombi were more unstable, leading to continuous embolization of platelet aggregates from the thrombus surface. Nonetheless, despite persistent surface embolization, a stable thrombus core eventually developed (typically within 3-4 minutes after injury) that was of sufficient stability to resist the detaching effects of rapid blood flow over a 15-minute observation period. Local infusion of blebbistatin resulted in rapid destabilization of the primary hemostatic plug, with near complete embolization of the formed thrombus (Figure 7A). Similarly, inhibition of Rho kinase produced a similar defect in the stability of the pri-mary hemostatic plug, with embolization occurring within 10 to 15 seconds of drug infusion (Figure 7A-C). In control studies, injection of the vehicle (DMSO) or the inactive blebbistatin enantiomer (control) had no adverse effect on the stability of the primary hemostatic plug (Figure 7A,C). Taken together, these findings suggest a major role for Rho kinase–dependent platelet contractility in maintaining the integrity of the primary hemostatic plug, independent of thrombin and fibrin polymers.
Role of myosin IIA and Rho kinase in regulating the stability of the primary hemostatic plug. Vascular injury was induced in the mesenteric postcapillary venules of anesthetized C57/Bl6 mice by needle puncture, in the presence of lepirudin (50 mg/kg, administered intravenously prior to injury). In the indicated experiments, the effects of blebbistatin, an inactive enantiomer of blebbistatin (control), vehicle (DMSO), or H1152 on thrombus stability was assessed following repetitive injections (denoted by ▀), with concentrations and volumes as described in “Intravital microscopy.” (A) The relative change in surface area of a given thrombus over time was determined off-line, and expressed relative to the initial surface area of the thrombus prior to injection. These results depict data taken from 1 of 4 independent experiments, with representative images from one such experiment depicted in panel B. (C) The percentage decrease in thrombus size following each infusion of vehicle/inhibitor was quantified as described in “Intravital microscopy,” and expressed as a percentage (%) of the original thrombus prior to infusion. These results represent the mean plus or minus SEM (n = 4), where *** indicates P < .001.
Role of myosin IIA and Rho kinase in regulating the stability of the primary hemostatic plug. Vascular injury was induced in the mesenteric postcapillary venules of anesthetized C57/Bl6 mice by needle puncture, in the presence of lepirudin (50 mg/kg, administered intravenously prior to injury). In the indicated experiments, the effects of blebbistatin, an inactive enantiomer of blebbistatin (control), vehicle (DMSO), or H1152 on thrombus stability was assessed following repetitive injections (denoted by ▀), with concentrations and volumes as described in “Intravital microscopy.” (A) The relative change in surface area of a given thrombus over time was determined off-line, and expressed relative to the initial surface area of the thrombus prior to injection. These results depict data taken from 1 of 4 independent experiments, with representative images from one such experiment depicted in panel B. (C) The percentage decrease in thrombus size following each infusion of vehicle/inhibitor was quantified as described in “Intravital microscopy,” and expressed as a percentage (%) of the original thrombus prior to infusion. These results represent the mean plus or minus SEM (n = 4), where *** indicates P < .001.
Discussion
The studies presented here demonstrate that the extracellular transmission of cytoskeletal contractile forces plays an important role in promoting thrombus contraction, independent of thrombin and fibrin formation. In contrast to fibrin clot retraction, platelet thrombus contraction appears to be principally regulated by Rho kinase–dependent signaling mechanisms. Furthermore, we have demonstrated that inhibition of thrombus contraction with blebbistatin or Rho kinase antagonists markedly undermines the stability of forming thrombi, leading to rapid embolization of the primary hemostatic plug. These studies suggest that during hemostasis platelets use a 2-stage contractile mechanism: (1) initially involving the Rho kinase–dependent contraction and maintenance of the primary hemostatic plug; (2) followed by fibrin generation and the calcium-dependent retraction of the secondary hemostatic plug.
The importance of the platelet contractile apparatus in supporting the hemostatic function of platelets is well established,4,8,22,37,38 with strong evidence that contractility is essential for platelet shape change7,8 and retraction of fibrin clots.11,12 However, the role of platelet contractility, in particular the extracellular transmission of cytoskeletal contractile forces, in regulating primary hemostasis has been less clearly defined. By using a new in vivo model of primary hemostasis, in which the vessel wall in the microcirculation is breached by standardized microinjector needles under experimental conditions preventing blood coagulation, we have been able to examine the role of contractility in regulating the primary hemostatic plug. A major advantage of this model is the ability to locally inject antagonists of contractility directly into the microcirculation. This has the advantages of minimizing potential secondary systemic effects of the compounds on the thrombotic process, that is, vasodilatory effects on vascular smooth muscle cells leading to alterations in blood pressure and blood flow; it also allows repetitive cyclic injections of compounds, thus enabling dynamic analysis of their effects on thrombus formation and stability. Importantly, this also allows accurate insight into the inherent instability of thrombi once contraction is blocked, as the time from inject to destabilization of thrombi can easily be determined. Finally, this model allows high-magnification imaging of forming mural thrombi, providing insight into the degree of packing of individual platelets and a direct analysis of the effects of contractility inhibitors on the thromboembolic process. Our ability to inject compounds into the microcirculation once thrombi have already formed suggest that any effects on thrombus stability are unlikely to be due to inhibition of platelet shape change, as this early platelet response is already likely to have occurred well before administration of inhibitors. Furthermore, our studies were performed under experimental conditions blocking blood coagulation, thus it is unlikely that the effects we observed in vivo were due to effects on fibrin clot retraction. This, combined with the demonstration that Rho kinase inhibitors induce rapid destabilization of the primary hemostatic plug, provide strong evidence that inhibition of thrombus contraction is likely to be the primary mechanism undermining the hemostatic plug.
A significant finding in the current study was the prominent role played by Rho kinase in thrombus contraction. Rho kinase has previously been demonstrated to play an important role in regulating the formation of focal adhesion–like complexes18 and actin stress fibers in spreading platelets,22 processes that presumably are important for sustaining platelet-matrix and platelet-platelet adhesive interactions under flow.23 Notably these actin-based structures typically require actomyosin-based contractility for their formation in the cell.39 Based on studies in nucleated cells, including fibroblasts, endothelial cells, and smooth muscle cells, Rho kinase–dependent contractility appears critical for the bundling of actin filaments, a process that applies tension to integrin bonds, inducing receptor clustering and recruitment of integrins into focal adhesion sites.40,41 A small number of Rho-dependent focal adhesion–like complexes develop in spread platelets; however, these structures do not appear to be essential for the transmission of contractile forces to fibrin polymers. It is unclear whether similar structures are involved in regulating platelet-platelet interactions, as focal adhesions do not typically form at sites of cell-cell adhesion.42 It is possible that Rho-dependent contractility of actin cables and subsequent integrin clustering is important to strengthen platelet-platelet adhesion contacts. The recent demonstration that mouse platelets lacking myosin IIA (ΔMYH mouse) have defective integrin outside-in signaling and reduced thrombus stability under flow15 is consistent with such a possibility. Presumably, high avidity adhesive interactions are be less critical for clot retraction, particularly when studied under nonsheared conditions,18 providing a potential explanation for the lack of involvement of Rho kinase in this process.
A hallmark feature of thrombus contraction is the tight packing of aggregated platelets, a process that may be critical for maintaining the stability of forming thrombi. It has long been recognized that the retraction of fibrin polymers by platelets markedly reduces the gap distance between adjacent platelets.43 Such close apposition of surface membranes is thought to facilitate the interactions of various adhesive and activation receptors on the platelet surface. These include the cell adhesion molecules ESAM, JAM-A, Sema4D, SLAM, and Ephrin/Eph kinases, some of which have recently been reported to sustain thrombus growth.15,44,45 The reduction in gap distance could also limit the diffusion of plasma molecules and secreted adhesive proteins46 within the developing aggregate and minimize the “wash-out” effects of blood flow on soluble agonists.44 As a consequence, the close apposition of adjacent platelets is likely to promote thrombus stability.44,45 Our findings are consistent with this concept, demonstrating that inhibition of thrombus contraction markedly undermines the tight packing of individual platelets, leading to destabilization of platelet thrombi. This was most apparent with blebbistatin, which prevented the formation of a tight core of aggregated platelets within developing thrombi. Rho inhibitors had a similar effects, although were generally less potent at inducing thrombus destabilization once tight aggregates had formed. This may be partly explained by the residual contractility induced by cytosolic calcium flux or alternatively, complete inhibition of myosin IIA may cause other functional defects, such as reduced granule release, that may enhance the observed inhibitory effects.
A striking effect in the in vivo models was the rapidity in which platelet thrombi embolize following exposure to inhibitors of platelet contractility, particularly under experimental conditions limiting thrombin generation and fibrin formation. It has long been known that platelet adhesion contacts within the primary hemostatic plug are intrinsically unstable, requiring fibrin generation to stabilize the formed aggregates to secure hemostasis.47,48 The in vivo results presented here indicate that in the absence of contractility, primary hemostatic plugs are extremely unstable, becoming detached from the site of injury within seconds of exposure to contractility inhibitors. These provocative findings suggests that there may be 2 distinct phases to stabilizing the primary hemostatic plug: the first is a rapid phase linked to platelet contractility and the physical tightening of platelet-platelet adhesion contacts, and the second, a slower phase linked to thrombin generation and fibrin polymerization. Such a 2-stage stabilization process provides a dynamic mechanism of temporal control of thrombus growth and stability. This not only has important implications for the understanding of hemostasis but may also lead to new approaches to regulate the size and stability of forming thrombi in vivo.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Shaun Coughlin for provision of PAR4-deficient mice. The authors also thank Drs Johan Heemskerk, Steve Watson, and Robert Andrews for helpful comments.
This work was funded by grants from the National Heart Foundation (NHF) of Australia and the National Health and Medical Research Council (NHMRC) of Australia. A.O. is the recipient of a joint NHMRC and NHF postgraduate scholarship.
Authorship
Contribution: A.O. designed and performed experiments, analyzed and interpreted data, prepared figures, and assisted with writing the manuscript; E.W. performed research and contributed a new analytical tool; S.H. performed research; W.N. contributed a new analytical tool; J.H. contributed an analytical tool; S.M.S. designed research, analyzed and interpreted data, performed statistical analysis, prepared figures, and drafted the manuscript; S.P.J. designed research, interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaun P. Jackson, Australian Centre for Blood Diseases, Monash University, 6th Floor Burnet Bldg, 89 Commercial Road, Melbourne, Victoria, Australia; e-mail: shaun.jackson@med.monash.edu.au.
References
Author notes
*S.M.S. and S.P.J. are equal senior authors.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal