In this issue of Blood, Paiva and colleagues report that MFC performed on bone marrow examinations on day 100 after autotransplantation and intended to provide a measure of MRD in multiple myeloma distinguished patients with strikingly different progression-free and overall survival, independent of standard baseline prognostic variables and IFx-defined CR.
Marked and rapid onset of response has been widely recognized as critically important for long-term prognosis and cure in leukemias (ie, chronic myeloid leukemia and acute promyelocytic leukemia) that lend themselves to minimal residual disease (MRD) analysis based on polymerase chain reaction. Because of the need for generating patient-specific primers, such molecular evaluation of complete response (CR) status has not been widely applied in myeloma,1 where response assessment relies on serial determinations of usually secreted monoclonal or M-protein. Rare with standard dose chemotherapy, the frequency of immunofixation (IFx)–defined CR approaches 50% with high-dose melphalan-based transplant regimens, doubling the median survival to beyond 6 years.2,3 Such high CR rates have recently been reported with the use of novel agents, although follow-up is too short to comment on the durability of such remissions.4 By employing high-resolution multiparameter flow cytometry (MFC), Paiva et al document MRD-negativity status in two-thirds of patients within 100 days after transplantation with significant survival advantage, regardless of IFx status.
A number of issues warrant discussion. (1) Patchy bone marrow involvement is a common feature of myeloma, readily appreciated by MRI and PET-CT scanning. In cases of predominantly macro-focal disease presentation, iliac crest sampling sites may be uninvolved and account for negative MFC results, despite an enormous tumor load residing in focal sites.5 In the typical myeloma patient, focal MRI lesions resolve slowly, lagging behind IFx-defined CR by up to several years; conversely, reappearance of focal lesions can antedate M-protein relapse. Focal MRI lesions matter clinically in that a greater number affects survival adversely while their resolution is associated with superior survival. Focal lesion–resident plasma cells express high levels of DKK1, suppressing Wnt signaling and, hence, osteoblast differentiation from mesenchymal stem cells.6 This novel mechanism of myeloma-related bone disease is a distinguishing feature vis-à-vis its precursor condition, monoclonal gammopathy of undetermined significance.
(2) The clinical relevance of IFx CR has been challenged recently.7 Thus, CR occurs in fewer than 10% of case myeloma, yet survival duration is not affected adversely. Conversely, despite high CR rates, remissions are seldom sustained, and survival is dismal in high-risk disease. MFC analysis may help distinguish whether the poor outcome is a consequence of less profound tumor cytoreduction or rapid regrowth of tumor clones with high proliferation potential. Such investigations could greatly impact clinical management.
(3) Gene expression profiling (GEP) analysis performed before initiation of therapy has been validated as an enormously powerful tool for outcome discrimination.8 By applying Total Therapy 3 that incorporated both thalidomide and bortezomib into a tandem transplant approach, the 4-year estimates of survival approached 90% in low-risk disease and were less than 40% in high-risk myeloma (P < .0001). Applied in the latter condition, MFC analysis may provide useful information, guiding the indications of type and duration of consolidation and maintenance therapies. Emerging data are consistent with the evolution of molecularly defined high-risk disease from low-risk status at diagnosis as a consequence of unequal expansion of 2 clonally related subpopulations that can be distinguished by 1q gains. The identification of cell-surface markers accurately discriminating low-risk and high-risk clones by MFC may provide critical information about the mechanism of disease escape.
(4) A further consideration relates to the importance of the bone marrow stroma that, in the case of myeloma, are not merely a target of the disease in terms of osteoclast activation and osteoblast inactivation, but also partake intimately and intrinsically in disease propagation and resistance to therapy. MFC analysis strictly examines the tumor cells. We have extended GEP investigations to bone marrow biopsy samples in order to examine myeloma-stroma interaction.
Where does this leave the reader in judging the usefulness of the Spanish group's contributions? The authors have made available a superb technique that permits the objective and quantitative assessment of residual malignant bone marrow plasma cells. Besides the prospect of distinguishing mechanisms of therapeutic failure in high-risk myeloma, MFC may be a valuable tool in low-risk myeloma for assessing MRD as an early surrogate endpoint for survival in trials that investigate the value of transplants versus strictly nontransplant-based novel agent combinations.
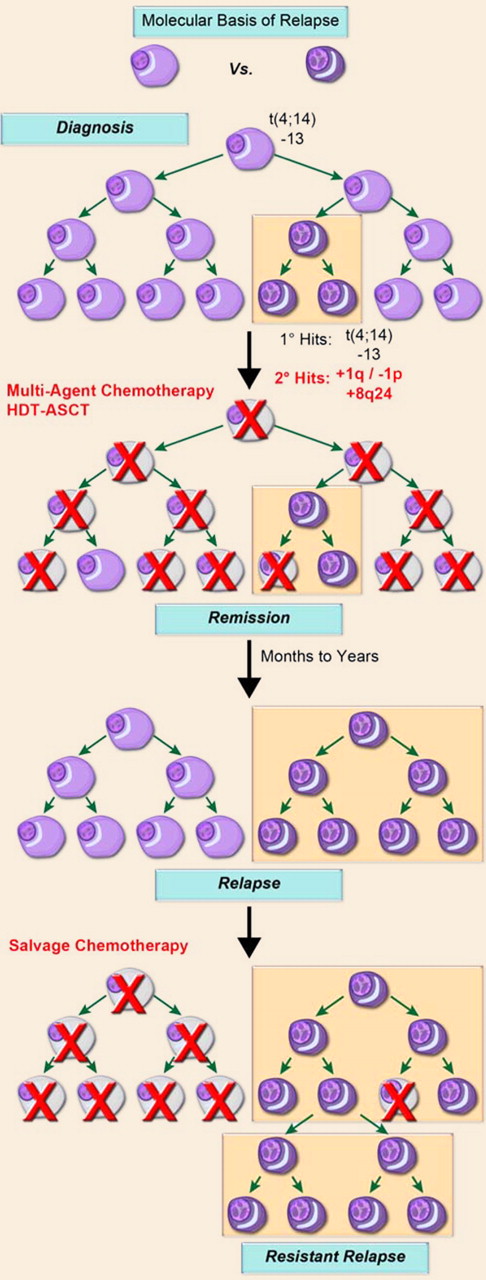
A model of disease progression. Multiple myeloma is characterized by the clonal expansion of malignant plasma cells driven by initiating genetic events, such as oncogene activating translocations, for example, t(4;14), hyperdiploidy, deletion of chromosome 13, and deletion of 17p13. During subclinical growth, an inherent genomic instability characteristic of these terminally differentiated cells leads to secondary genetic events, such as gains of chromosome 1q and deletion of 1p as well as gains of 8q24, that provide a growth and/or survival advantage to a subpopulation of cells. A gene expression–based signature, reflective of a minimum proportion of cells with these secondary lesions, can define high-risk disease. Following therapy, such as stem-cell supported high-dose melphalan, a sizeable portion of patients achieve complete remission that can be monitored by MFC. Toward relapse, both the percentage of tumor cells with gains of chromosome 1q and molecular risk score invariably increase. These data suggest that a subpopulation of high-risk cells survives therapy and eventually contributes to progressively resistant relapses. The use of MFC to measure MRD, as reported by Paiva et al, appears well suited for the identification of cell-surface marker(s) discriminating tumor subpopulations, exhibiting differential cytoreduction and regrowth kinetics, with significant impact on the clinical management of the disease.
A model of disease progression. Multiple myeloma is characterized by the clonal expansion of malignant plasma cells driven by initiating genetic events, such as oncogene activating translocations, for example, t(4;14), hyperdiploidy, deletion of chromosome 13, and deletion of 17p13. During subclinical growth, an inherent genomic instability characteristic of these terminally differentiated cells leads to secondary genetic events, such as gains of chromosome 1q and deletion of 1p as well as gains of 8q24, that provide a growth and/or survival advantage to a subpopulation of cells. A gene expression–based signature, reflective of a minimum proportion of cells with these secondary lesions, can define high-risk disease. Following therapy, such as stem-cell supported high-dose melphalan, a sizeable portion of patients achieve complete remission that can be monitored by MFC. Toward relapse, both the percentage of tumor cells with gains of chromosome 1q and molecular risk score invariably increase. These data suggest that a subpopulation of high-risk cells survives therapy and eventually contributes to progressively resistant relapses. The use of MFC to measure MRD, as reported by Paiva et al, appears well suited for the identification of cell-surface marker(s) discriminating tumor subpopulations, exhibiting differential cytoreduction and regrowth kinetics, with significant impact on the clinical management of the disease.
Conflict-of-interest disclosure: B.B. serves on research advisory boards for Celgene and Millennium corporations, and his institution has received data management support from both companies; J.D.S. serves on an advisory board for Novartis and has received honoraria from that company; the remaining author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal