Abstract
We used the thrombin generation assay to evaluate the hypercoagulable state according to JAK2V617F mutational status in essential thrombocythemia (ET) and polycythemia vera (PV) patients. Thrombin generation was determined in the presence and absence of activated protein C (APC), and APC resistance was expressed as normalized APC sensitivity ratio (nAPCsr). Tissue factor pathway inhibitor (TFPI), total and free protein S (PS), prothrombin (FII), factor V (FV), and neutrophil elastase were measured in plasma; CD11b was measured on neutrophils. Compared with normal controls, patients had a lower endogenous thrombin potential in the absence of APC but had a higher endogenous thrombin potential in the presence of APC, showing the occurrence of APC resistance. The nAPCsr increased in JAK2V617F carriers compared with noncarriers and was highest in JAK2V617F homozygous patients. FII, FV, free PS, and TFPI levels were reduced in patients, mainly in JAK2V617F carriers. Multiple regression analysis indicated the low free PS level as major determinant of the increased nAPCsr. Elastase was increased in patients and inversely correlated with free PS. In conclusion, these data indicate the occurrence of acquired APC resistance in ET and PV patients, probably because of a reduction in free PS levels. The APC-resistant phenotype is influenced by the JAK2V617F mutational load.
Introduction
Essential thrombocythemia (ET) and polycythemia vera (PV) are 2 of the chronic myeloproliferative disorders (MPDs), a group of clonal hematopoietic diseases characterized by increased production of leukocytes, erythrocytes, and/or platelets.1 The clinical course of ET and PV is characterized by an increased frequency of thrombotic complications, which may occur at venous, arterial, and microcirculatory sites.2 Although advanced age and history of thrombosis are well-identified independent predictors of recurrent thrombosis, the origin of the hypercoagulable state in MPD patients is still unclear. Numerous mechanisms, including blood hyperviscosity and quantitative/qualitative abnormalities of blood cells, have been advocated.3 Recently, leukocytosis has been identified as an additional risk factor for thrombosis in both ET and PV patients.4-7
Approximately 50% of ET patients and almost all PV patients carry the somatic V617F mutation in the Janus 2 tyrosine kinase (JAK2) gene, which leads to a constitutive activation of this enzyme in platelets and neutrophils.8-10 Clinical data indicate that the JAK2V617F mutation is associated with an increased thrombotic risk in ET and PV patients.11-14 Laboratory data show that neutrophils and platelets circulate in an activated status in these patients, particularly in those carrying the JAK2V617F mutation.15-19 In vitro studies have shown that both activated neutrophils and platelets can affect the hemostatic balance. Specifically, activated neutrophils can impair blood coagulation by releasing intragranule-associated proteases (ie, elastase and cathepsin G), which are known to degrade numerous inhibitors of coagulation, including protein S (PS),20 protein C (PC),21 and tissue factor pathway inhibitor (TFPI),22 as well as coagulation factors.23 Activated platelets directly participate in thrombin generation in multiple ways.24 In ET and PV patients, in vivo blood cell activation is associated with the detection of elevated plasma markers of hypercoagulation,25,26 suggesting the presence of an acquired thrombophilia secondary to cell activation.
To investigate the influence of blood cell activation on the hemostatic balance of MPD patients, we measured in this study the thrombin generation by the calibrated automated thrombogram method in plasma from ET and PV patients. This assay reflects the net results of the procoagulant and anticoagulant forces operating in plasma. The area under the thrombin generation curve (also known as the endogenous thrombin potential [ETP]) is a good overall indicator of prothrombotic and hemorrhagic tendency.27,28 In addition, to explore possible dysfunction of the PC/PS anticoagulant pathway, the thrombin generation test was also performed in the presence of activated PC (APC). This setup was chosen for its sensitivity to both genetic and acquired hypercoagulable states.29-31
Thrombin generation parameters determined under different experimental conditions were evaluated in relation to platelet and neutrophil cell counts, leukocyte activation markers (ie, surface expression of CD11b and plasma elastase levels), JAK2V617F mutational status, and the levels of coagulation factors (prothrombin [FII] and factor V [FV]) and inhibitors (total and free PS and TFPI) that are major determinants of the thrombin generation assay.32
This is the first report describing thrombin generation in patients with ET and PV.
Methods
Study population
From January 2006 to July 2007, 59 ET patients (18 males and 41 females; age range, 28-83 years) and 30 PV patients (11 females and 19 males; age range, 18-80 years) were consecutively enrolled at the Hematology Department of Bergamo Hospital (Italy), after giving informed consent. ET and PV patients were diagnosed according to the Polycythemia Vera Study Group criteria.33 Fifteen patients with ET (25%) and 12 with PV (40%) had positive history of thrombotic events (venous or arterial events). Sixty healthy subjects (18 males and 42 females; age range, 30-75 years) without history of thrombohemorrhagic events acted as a control group; none of them had symptoms of active infection or inflammatory disease. All investigations were approved by the local ethics committee (Comitato di Bioetica, Ospedali Riuniti, Bergamo, Italy). The procedures were in accordance with the Declaration of Helsinki of 1975, as revised in 2000, and blood samples were obtained with the subjects' informed consent. None of the study subjects was taking oral contraceptives or hormone replacement therapy at the time of blood collection.
Blood collection and plasma preparation
Blood samples were drawn early in the morning, before any therapy, with a 21-gauge butterfly needle after applying a light tourniquet. After discarding the first 3 mL, blood was collected into sterile siliconized tubes containing K3-ethylenediamine tetraacetic acid for molecular biology studies, or trisodium citrate (0.129 M, 1:9 vol:vol) for coagulation and cytofluorimetric studies. Platelet-poor plasma was isolated from citrated blood by double centrifugation at 3000g for 15 minutes at room temperature, aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C until testing.
Routine hematologic assays
White blood cell differential count, hematocrit, hemoglobin, red blood cell count, and platelet count were determined by an NE800 Analyzer (Dasit, Milan, Italy).
Thrombin generation assay
Thrombin generation was determined in platelet-poor plasma by calibrated automated thrombography.27 This method uses a low-affinity fluorogenic substrate for thrombin to monitor the formation and inhibition of thrombin in a plasma sample triggered with tissue factor (TF), phospholipids, and CaCl2. The assay was performed in the absence and presence of APC, essentially as described.34 Briefly, 80 μL plasma was pipetted into the well of a microtiter plate together with 15 μL of a mixture of TF (Innovin; Dade Behring, Deerfield, IL) and synthetic phospholipid vesicles (DOPS/DOPC/DOPE, 20/60/20 mol/mol/mol). After 5 minutes of prewarming at 37°C, 10 μL buffer or purified APC (Innovative Research, Southfield, MI) was added to the respective wells, and the reaction was started with 20 μL of a fluorogenic substrate (Z-Gly-Gly-Arg-AMC; Bachem, Bubendorf, Switzerland)/CaCl2 mixture. Final concentrations were 6.8 pM TF, 30 μM phospholipids, 16 mM added CaCl2, and 300 μM fluorogenic substrate. The APC concentration (∼4 nM) was chosen to inhibit thrombin generation in normal pooled plasma by 90%. Fluorescence was read in a Fluoroskan Ascent reader (Thermo Labsystems, Helsinki, Finland), and thrombin generation curves were calculated using Thrombinoscope software (Thrombinoscope, Maastricht, The Netherlands).
Thrombin generation curves were described in terms of lag time, peak height, and area under the curve (ETP). In addition, a normalized APC sensitivity ratio (nAPCsr) was calculated for each plasma by dividing the ratio of the ETPs obtained in the presence and absence of APC (ETP+APC/ETP−APC) by the same ratio calculated in normal pooled plasma in the same experiment. This ETP-based nAPCsr varies between 0 and 10 and increases with increasing APC resistance.
Activated partial thromboplastin time-based APC resistance test
The activated partial thromboplastin time (aPTT)-based APC resistance test was performed in undiluted plasma using the Coatest APC Resistance Kit (Chromogenix, Mölndal, Sweden) in an ACL 300 Research coagulometer (Automated Coagulation Laboratory, Instrumentation Laboratory, Milan, Italy) following the manufacturer's instructions. Plasmas from homozygous and heterozygous carriers of the factor V Leiden mutation were included as positive controls. Results were expressed as the ratio of the clotting times measured in the presence and absence of APC (APC sensitivity ratio [APCsr]). The aPTT-based APCsr is more than or equal to 1 and decreases with increasing APC resistance.
Plasma levels of coagulation factors and inhibitors
The plasma levels of FII, FV, PS, and TFPI were measured in all study subjects. FII levels were determined by a chromogenic assay after complete activation of FII with Ecarin in 1:500-diluted plasma.34 FV levels were measured by a prothrombinase-based assay after complete activation of FV with thrombin in 1:1000-diluted plasma.34 Total PS antigen levels were quantified by homemade enzyme-linked immunosorbent assay, as previously described.35 Free-PS and free-TFPI antigen levels were quantified with commercial enzyme-linked immunosorbent assay kits (Asserachrom; Diagnostica Stago, Asnières sur Seine, France) according to the manufacturer's instructions.
Neutrophil CD11b and plasma neutrophil elastase
To assess neutrophil activation, the expression of CD11b on neutrophil membrane and plasma levels of neutrophil elastase were evaluated as described.17
For CD11b measurement, 50 μL fresh whole blood was incubated for 20 minutes in the dark with saturating concentrations of phycoerythrin-conjugated anti-CD11b or irrelevant isotype-identical negative control MoAbs (IgG2a-phycoerythrin; BD Biosciences, San Jose, CA). After incubation, the erythrocytes were lysed, and the samples were centrifuged (5 minutes at 580g), resuspended in phosphate-buffered saline, and immediately analyzed by FACSCalibur. Neutrophils were selectively gated using their forward- and side-scatter properties, and 5000 neutrophil-gated events were measured for each sample. Results are expressed as mean fluorescence intensity (MFI) units.
Neutrophil elastase was quantified in plasma by an immunoassay procedure (PMN Elastase IMAC; Merck, Darmstadt, Germany).
DNA analysis
Genomic DNA was extracted from K3-ethylenediamine tetraacetic acid anticoagulated blood samples using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN). FV-Leiden and FII G20210A mutations were detected with LightCycler-Factor V Leiden and LightCycler-Prothrombin G20210A mutation detection kits (Roche Molecular Diagnostics, Mannheim, Germany), respectively. All reactions were carried out with the Roche LightCycler 24 and analyzed with the LightCycler software, version 4.05. The allele-specific polymerase chain reaction for JAK2V617F mutation was performed as previously described.26 To discriminate between homozygous and heterozygous mutation carriers, polymerase chain reaction products were digested with the restriction enzyme BsaXI as described by Baxter et al.9
Statistical analysis
Data were compared between groups using the Student t test or the nonparametric Mann-Whitney-Wilcoxon U test, according to the distribution of the test variable. Linear regression analysis was used to test the association between continuous variables. Multiple regression analysis was used to correct for differences in age and sex distribution, JAK2V617F mutational status, and pharmacologic treatment among groups. The individual effects of demographic and clinical variables, as well as plasma levels of coagulation factors and anticoagulant proteins, on thrombin generation parameters were also assessed by multiple regression analysis. Regression coefficients are expressed as B, which represents the absolute change of the dependent variable when the independent variable increases by 1 unit. Statistical analysis was performed with the SPSS 12.0 statistical package (SPSS, Chicago, IL).
Results
Characteristics of the study population
The study population consisted of 59 subjects with ET, 30 subjects with PV, and 60 healthy controls. Their general characteristics are presented in Table 1. Two-thirds of ET patients were female, whereas among PV patients there were slightly more males than females. Platelet and leukocyte counts were elevated in both ET and PV patients compared with controls, whereas the erythrocyte count was decreased in ET patients and increased in PV patients. At the time of enrollment, all but 7 patients were receiving antiplatelet and/or cytoreductive therapies, such as, aspirin (20 ET and 13 PV patients), hydroxyurea (10 ET patients), hydroxyurea plus aspirin (21 ET and 17 PV patients), and busulphan plus aspirin (1 ET patient). Therefore, 54% of ET patients and 56% of PV patients were on a cytoreductive treatment. None of the ET patients and 3 PV patients were heterozygous carriers of the FV Leiden mutation; 2 ET patients and 1 control were heterozygous carriers of the FII G20210A mutation. Thirty ET patients (50.8%) and 27 PV patients (90%) were positive for the JAK2V617F mutation. Among ET patients, all JAK2V617F carriers were heterozygous, whereas among PV patients, there were 17 heterozygous and 10 homozygous carriers.
Characteristics of the study subjects
| . | Controls . | ET . | JAK2V617F . | PV . | JAK2V617F . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative . | Heterozygous . | P . | Negative . | Heterozygous . | Homozygous . | P . | ||||
| n | 60 | 59 | 29 | 30 | 30 | 3 | 17 | 10 | ||
| Male/female | 18/42 | 18/41 | 10/19 | 8/22 | 19/11 | 2/1 | 12/5 | 5/5 | ||
| Age, y (range) | 57 (27-75) | 59 (23-82) | 56 (30-78) | 65 (28-82) | 59 (19-80) | 52 (18-77) | 59 (35-80) | 67 (51-78) | ||
| Platelets, ×109/L (range) | 258 (149-428) | 679* (319-1744) | 722* (335-1601) | 638* (319-1744) | NS | 510* (164-1085) | 571 (400-670) | 557* (261-1085) | 418* (164-954) | NS |
| White blood cells, ×109/L (range) | 6.84 (4.32-10.3) | 7.6* (3.40-17.8) | 7.31 (3.4-11.6) | 7.99* (4.6-17.8) | NS | 10.24* (3.45-36.21) | 7.76 (6.3-10.4) | 9.33* (3.61-23.82) | 12.4* (3.45-36.21) | NS |
| Neutrophils, ×109/L (range) | 3.94 (3.85-6.68) | 4.7* (1.7-12.46) | 4.42 (1.7-8.3) | 5.07* (2.5-12.46) | NS | 7.17* (1.78-30.8) | 4.72 (3.59-6.42) | 6.30* (1.78-15.48) | 9.5* (2.5-30.8) | NS |
| Red blood cells, ×109/L (range) | 4.96 (4.17-5.66) | 4.54* (3.04-5.88) | 4.7 (3.04-5.88) | 4.38* (3.18-5.75) | NS | 5.72* (3.85-7.24) | 6.15 (5.1-7.2) | 5.62* (4.03-7.24) | 5.67* (3.85-6.68) | NS |
| History of thrombosis | — | 15 (25%) | 7 (24%) | 8 (27%) | 12 (40%) | — | 4 (23%) | 8 (80%) | ||
| . | Controls . | ET . | JAK2V617F . | PV . | JAK2V617F . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative . | Heterozygous . | P . | Negative . | Heterozygous . | Homozygous . | P . | ||||
| n | 60 | 59 | 29 | 30 | 30 | 3 | 17 | 10 | ||
| Male/female | 18/42 | 18/41 | 10/19 | 8/22 | 19/11 | 2/1 | 12/5 | 5/5 | ||
| Age, y (range) | 57 (27-75) | 59 (23-82) | 56 (30-78) | 65 (28-82) | 59 (19-80) | 52 (18-77) | 59 (35-80) | 67 (51-78) | ||
| Platelets, ×109/L (range) | 258 (149-428) | 679* (319-1744) | 722* (335-1601) | 638* (319-1744) | NS | 510* (164-1085) | 571 (400-670) | 557* (261-1085) | 418* (164-954) | NS |
| White blood cells, ×109/L (range) | 6.84 (4.32-10.3) | 7.6* (3.40-17.8) | 7.31 (3.4-11.6) | 7.99* (4.6-17.8) | NS | 10.24* (3.45-36.21) | 7.76 (6.3-10.4) | 9.33* (3.61-23.82) | 12.4* (3.45-36.21) | NS |
| Neutrophils, ×109/L (range) | 3.94 (3.85-6.68) | 4.7* (1.7-12.46) | 4.42 (1.7-8.3) | 5.07* (2.5-12.46) | NS | 7.17* (1.78-30.8) | 4.72 (3.59-6.42) | 6.30* (1.78-15.48) | 9.5* (2.5-30.8) | NS |
| Red blood cells, ×109/L (range) | 4.96 (4.17-5.66) | 4.54* (3.04-5.88) | 4.7 (3.04-5.88) | 4.38* (3.18-5.75) | NS | 5.72* (3.85-7.24) | 6.15 (5.1-7.2) | 5.62* (4.03-7.24) | 5.67* (3.85-6.68) | NS |
| History of thrombosis | — | 15 (25%) | 7 (24%) | 8 (27%) | 12 (40%) | — | 4 (23%) | 8 (80%) | ||
The data are tabulated according to disease type (ET or PV) and to JAK2V617F mutational status. Data presented as number for sex and history of thrombosis, mean (range) for the other parameters.
NS indicates not significant.
P < .05 versus controls.
Thrombin generation and ETP-based APC resistance
To characterize the overall coagulation phenotype of ET and PV patients, thrombin generation was determined in platelet-poor plasma from patients and controls after initiating coagulation with 6.8 pM TF in the absence and presence of 4 nM APC. Results are summarized in Table 2.
Thrombin generation parameters
| . | −APC . | +APC . | ||||||
|---|---|---|---|---|---|---|---|---|
| Lag time, minutes . | ETP, nM · min . | Peak, nM . | Peak time, minutes . | Lag time, minutes . | ETP, nM · min . | Peak, nM . | Peak time, minutes . | |
| Controls | 1.93 ± 0.34 | 821 ± 118 | 248 ± 34 | 3.78 ± 0.47 | 2.30 ± 0.35 | 211 ± 102 | 61 ± 30 | 4.29 ± 0.57 |
| ET | 1.94 ± 0.33 | 760 ± 177* | 242 ± 54 | 3.63 ± 0.43 | 2.31 ± 0.37 | 286 ± 152† | 81 ± 45† | 4.31 ± 0.59 |
| Negative (n = 29) | 1.96 ± 0.31 | 800 ± 168 | 249 ± 58 | 3.71 ± 0.4 | 2.34 ± 0.39 | 255 ± 150 | 72 ± 48 | 4.39 ± 0.63 |
| Heterozygous (n = 30) | 1.91 ± 0.35 | 716 ± 179† | 236 ± 49 | 3.56 ± 0.46* | 2.29 ± 0.35 | 316 ± 150† | 89 ± 42† | 4.23 ± 0.57 |
| PV | 1.88 ± 0.36 | 725 ± 166† | 235 ± 51 | 3.53 ± 0.58* | 2.24 ± 0.45 | 311 ± 214† | 92 ± 62† | 4.15 ± 0.59 |
| Negative (n = 3) | 2.01 ± 0.08 | 856 ± 288 | 274 ± 84 | 3.70 ± 0.01 | 2.26 ± 0.25 | 375 ± 202 | 110 ± 104 | 4.22 ± 0.18 |
| Heterozygous (n = 17) | 1.87 ± 0.42 | 727 ± 88† | 228 ± 41 | 3.61 ± 0.73* | 2.20 ± 0.46 | 273 ± 199† | 82 ± 60† | 4.14 ± 0.67 |
| Homozygous (n = 10) | 1.85 ± 0.32 | 684 ± 217† | 236 ± 56 | 3.38 ± 0.40† | 2.30 ± 0.51 | 352 ± 202† | 103 ± 55† | 4.14 ± 0.55 |
| . | −APC . | +APC . | ||||||
|---|---|---|---|---|---|---|---|---|
| Lag time, minutes . | ETP, nM · min . | Peak, nM . | Peak time, minutes . | Lag time, minutes . | ETP, nM · min . | Peak, nM . | Peak time, minutes . | |
| Controls | 1.93 ± 0.34 | 821 ± 118 | 248 ± 34 | 3.78 ± 0.47 | 2.30 ± 0.35 | 211 ± 102 | 61 ± 30 | 4.29 ± 0.57 |
| ET | 1.94 ± 0.33 | 760 ± 177* | 242 ± 54 | 3.63 ± 0.43 | 2.31 ± 0.37 | 286 ± 152† | 81 ± 45† | 4.31 ± 0.59 |
| Negative (n = 29) | 1.96 ± 0.31 | 800 ± 168 | 249 ± 58 | 3.71 ± 0.4 | 2.34 ± 0.39 | 255 ± 150 | 72 ± 48 | 4.39 ± 0.63 |
| Heterozygous (n = 30) | 1.91 ± 0.35 | 716 ± 179† | 236 ± 49 | 3.56 ± 0.46* | 2.29 ± 0.35 | 316 ± 150† | 89 ± 42† | 4.23 ± 0.57 |
| PV | 1.88 ± 0.36 | 725 ± 166† | 235 ± 51 | 3.53 ± 0.58* | 2.24 ± 0.45 | 311 ± 214† | 92 ± 62† | 4.15 ± 0.59 |
| Negative (n = 3) | 2.01 ± 0.08 | 856 ± 288 | 274 ± 84 | 3.70 ± 0.01 | 2.26 ± 0.25 | 375 ± 202 | 110 ± 104 | 4.22 ± 0.18 |
| Heterozygous (n = 17) | 1.87 ± 0.42 | 727 ± 88† | 228 ± 41 | 3.61 ± 0.73* | 2.20 ± 0.46 | 273 ± 199† | 82 ± 60† | 4.14 ± 0.67 |
| Homozygous (n = 10) | 1.85 ± 0.32 | 684 ± 217† | 236 ± 56 | 3.38 ± 0.40† | 2.30 ± 0.51 | 352 ± 202† | 103 ± 55† | 4.14 ± 0.55 |
The results (mean ± SD) obtained in the absence and presence of APC are shown according to disease type (ET or PV) and JAK2V617F mutational status.
P < .05 versus controls.
P < .01 versus controls.
In the absence of APC, the lag time of thrombin generation was not different between patients and controls, whereas the ETP was lower in patients than in controls. The ETP decreased progressively from controls to ET patients and to PV patients, and the reduction was more pronounced in patients carrying the JAK2V617F mutation. A similar trend was observed for the peak height of thrombin generation curves, but differences did not reach statistical significance. The time to peak was significantly shorter in JAK2V617F-positive ET and PV patients compared with controls.
The addition of APC to plasma markedly inhibited thrombin generation in both patients and controls, but patient plasma appeared to be less sensitive to the anticoagulant action of APC. Hence, the ETP and peak height obtained in the presence of APC were higher in MPD patients than in normal controls. Both ETP and peak height increased progressively from normal controls to ET patients and PV patients. The difference in ETP and peak height between ET patients and controls was mostly driven by the JAK2V617F carriers.
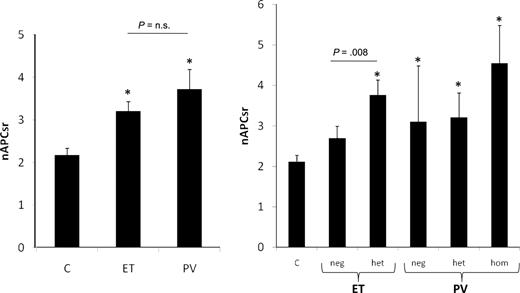
As a consequence of the reduced ETP in the absence of APC and the increased ETP in the presence of APC, MPD patients displayed a higher nAPCsr (3.37 ± 1.97) compared with controls (2.17 ± 0.92; P < .001), which indicates that their plasma is APC-resistant (Figure 1 left panel). PV patients were more APC-resistant (nAPCsr 3.72 ± 2.42) than ET patients (nAPCsr 3.20 ± 1.70), and APC resistance was more pronounced in JAK2V617F carriers, reaching the highest values in homozygous PV subjects (nAPCsr 4.55 ± 2.78; Figure 1 right panel). Excluding from the analysis the 3 PV patients with the FV Leiden mutation and the 2 ET patients with the FII G20210A mutation did not substantially modify these results.
ETP-based nAPCsr in controls and ET and PV patients. ETP-based nAPCsr are shown according to disease type (left panel) and to JAK2V617F mutational status (right panel). C indicates controls; Neg, negative; Het, heterozygous; and Hom, homozygous. *P < .01 versus controls.
ETP-based nAPCsr in controls and ET and PV patients. ETP-based nAPCsr are shown according to disease type (left panel) and to JAK2V617F mutational status (right panel). C indicates controls; Neg, negative; Het, heterozygous; and Hom, homozygous. *P < .01 versus controls.
MPD patients with a history of thrombosis (arterial or venous), presented with significant higher thrombin generation peak height values (261 ± 53 nM) than patients without thrombosis (230 ± 50 nM; P = .009). In addition, MPD patients who had experienced venous thrombosis, but not those with arterial thrombosis, had also, both in the absence and presence of APC, significantly higher ETP (−APC: 867 ± 58 vs 730 ± 18 nM · min, P = .014; +APC: 428 ± 62 vs 275 ± 18 nM · min, P = .006) and peak height values (−APC: 280 ± 21 vs 233 ± 5.4 nM, P = .005; +APC: 121.2 ± 20 vs 79.7 ± 5.3 nM, P = .011).
Because the 3 study groups (ie, controls, ET patients, and PV patients) differed in age distribution and sex ratio, and because JAK2V617F mutational status and pharmacologic treatment may have influenced thrombin generation in patients, multiple regression analysis was used to correct for these variables. Correction for these variable revealed that the observed differences in nAPCsr between all MPD patients and controls were largely explained by the JAK2V617F mutation (Table 3).
Influence of demographic and clinical variables on the ETP-based nAPCsr
| Variable . | B . | P . |
|---|---|---|
| Sex | 0.083 | .855 |
| Age | 0.004 | .808 |
| Hydroxyurea | −0.856 | .090 |
| Aspirin | −0.574 | .261 |
| JAK2V617F | 1.189 | .015 |
| Platelet count | 0.000 | .728 |
| White blood cell count | 0.055 | .279 |
| Hematocrit | 0.945 | .163 |
| Variable . | B . | P . |
|---|---|---|
| Sex | 0.083 | .855 |
| Age | 0.004 | .808 |
| Hydroxyurea | −0.856 | .090 |
| Aspirin | −0.574 | .261 |
| JAK2V617F | 1.189 | .015 |
| Platelet count | 0.000 | .728 |
| White blood cell count | 0.055 | .279 |
| Hematocrit | 0.945 | .163 |
Multiple regression analysis included sex (male = 0; female = 1), age, disease treatment (no = 0; yes = 1), history of thrombosis (no = 0; yes = 1), JAK2V617F mutational status (negative = 0; positive = 1), platelet and white blood cell counts, and hematocrit as independent variables and the ETP-based nAPCsr as the dependent variable.
aPTT-based APC resistance
To get more insight into the APC resistance phenotype of MPD patients, we also measured APC resistance with the classic aPTT-based assay in a random subset of patients (n = 43, 30 ET and 13 PV patients) and controls (n = 27). One PV patient was a carrier of the FV Leiden mutation. The clotting time in the absence of APC was slightly prolonged in ET (34.3 ± 3.7 seconds; P = .460) and PV patients (36.1 ± 2.9 seconds; P = .034), compared with controls (33.6 ± 3.7 seconds). In the presence of APC, the clotting time was 97.1 ± 15.2 seconds in ET, 106.7 ± 20.2 seconds in PV, and 101.9 ± 13.3 seconds in control subjects. The calculated APCsr was not significantly different between normal controls (3.04 ± 0.30) and ET patients (2.93 ± 0.46; P = .260), whereas a significantly lower APCsr was found in PV patients (2.83 ± 0.36; P = .039). However, after the exclusion of the FV Leiden–positive patients, the difference between PV and controls was not significant anymore (2.89 ± 0.23; P = .101). No differences were observed in the clotting times or aPTT-based APCsr between carriers and noncarriers of the JAK2V617F mutation. In the study population as a whole, there was a significant correlation between the ETP-based and the aPTT-based nAPCsr (R2 = −0.381, P = .001). Separate analysis of the 3 subgroups indicated that this correlation was driven mainly by ET patients, where the correlation was even stronger (R2 = −0.473, P = .008).
Plasma levels of coagulation factors and inhibitors
In an attempt to account for MPD-associated APC resistance, the plasma levels of FII, FV, total and free PS, and free TFPI were measured in all study subjects (Table 4). FII, FV, and free PS levels were significantly lower in MPD patients than in controls. When the data were analyzed according to disease type (ET or PV) and JAK2V617F mutational status (negative or positive), ET patients showed a significant reduction of FV and free PS compared with controls. The lower levels of these coagulation proteins were determined in ET patients by the JAK2V617F-positive subgroup of subjects who also had significantly reduced total PS compared with controls. In PV patients, a significant reduction of FII, FV, and total and free PS was present, which was more pronounced in the JAK2V617F homozygous subgroup. No significant differences were found in plasma-free TFPI concentrations. The plasma levels of FV showed a significant inverse correlation with the platelet count (R = −0.327; P = .002), whereas the plasma levels of total PS (R = −0.289; P = .007), free protein S (R = −0.325; P = .003), and FV (R = −229; P = .036) were all inversely correlated with the white blood cell count.
Plasma levels of coagulation factors and inhibitors
| . | FII, percentage . | FV, percentage . | Total PS, percentage . | Free PS, percentage . | Free TFPI, ng/mL . |
|---|---|---|---|---|---|
| Controls | 92.0 ± 14.7 | 94.9 ± 19.3 | 98.7 ± 16.6 | 89.8 ± 13.3 | 9.5 ± 3.1 |
| ET | 91.5 ± 17.4 | 85.3 ± 23.9* | 96.6 ± 19.6 | 83.6 ± 14.8* | 11.1 ± 4.0 |
| Negative (n = 29) | 95.0 ± 18.5 | 91.3 ± 26.0 | 101.0 ± 20 | 88.8 ± 15.5 | 11.5 ± 4.3 |
| Heterozygous (n = 30) | 87.8 ± 15.6 | 79.0 ± 19† | 92.1 ± 18.5* | 78.6 ± 11.5† | 10.7 ± 3.9 |
| PV | 82.7 ± 15† | 73.4 ± 21.1† | 85.9 ± 16† | 73.0 ± 13.8† | 10.0 ± 2.3 |
| Negative (n = 3) | 100 ± 23 | 80 ± 22 | 87.0 ± 11 | 75.1 ± 13 | 9.03 ± 2.8 |
| Heterozygous (n = 17) | 83 ± 12* | 80 ± 24† | 88.4 ± 17* | 74.5 ± 16† | 11.03 ± 2.8 |
| Homozygous (n = 10) | 78 ± 14† | 63 ± 14† | 83.1 ± 15† | 73.8 ± 10† | 8.83 ± 0.78 |
| . | FII, percentage . | FV, percentage . | Total PS, percentage . | Free PS, percentage . | Free TFPI, ng/mL . |
|---|---|---|---|---|---|
| Controls | 92.0 ± 14.7 | 94.9 ± 19.3 | 98.7 ± 16.6 | 89.8 ± 13.3 | 9.5 ± 3.1 |
| ET | 91.5 ± 17.4 | 85.3 ± 23.9* | 96.6 ± 19.6 | 83.6 ± 14.8* | 11.1 ± 4.0 |
| Negative (n = 29) | 95.0 ± 18.5 | 91.3 ± 26.0 | 101.0 ± 20 | 88.8 ± 15.5 | 11.5 ± 4.3 |
| Heterozygous (n = 30) | 87.8 ± 15.6 | 79.0 ± 19† | 92.1 ± 18.5* | 78.6 ± 11.5† | 10.7 ± 3.9 |
| PV | 82.7 ± 15† | 73.4 ± 21.1† | 85.9 ± 16† | 73.0 ± 13.8† | 10.0 ± 2.3 |
| Negative (n = 3) | 100 ± 23 | 80 ± 22 | 87.0 ± 11 | 75.1 ± 13 | 9.03 ± 2.8 |
| Heterozygous (n = 17) | 83 ± 12* | 80 ± 24† | 88.4 ± 17* | 74.5 ± 16† | 11.03 ± 2.8 |
| Homozygous (n = 10) | 78 ± 14† | 63 ± 14† | 83.1 ± 15† | 73.8 ± 10† | 8.83 ± 0.78 |
Data (mean ± SD) are reported according to disease type (ET or PV) and to JAK2V617F mutational status.
P< .05 versus controls.
P < .01 versus controls.
Correlation analyses between thrombin generation and coagulation factors
The effects of individual coagulation factor levels on the nAPCsr were investigated in the whole cohort of study subjects. A significant inverse correlation existed between the nAPCsr and the plasma levels of FV, total and free PS, and free TFPI. The strongest correlation was between nAPCsr and free protein S (Figure 2). After correction for age and sex by multiple regression analysis (Table 5), the levels of FV and of free PS still remain significant determinants of the nAPCsr.
Correlation between nAPCsr and free PS in controls and ET and PV patients. The nAPCsr is plotted against the free PS plasma concentration (%). R2 = 0.32; P < .001.
Correlation between nAPCsr and free PS in controls and ET and PV patients. The nAPCsr is plotted against the free PS plasma concentration (%). R2 = 0.32; P < .001.
Plasma determinants of nAPCsr in controls and ET and PV patients
| Variable . | B . | P . |
|---|---|---|
| Sex | −0.412 | .216 |
| Age | 0.016 | .169 |
| FII | −0.205 | .860 |
| FV | −2.695 | .001 |
| Total PS | 0.005 | .690 |
| Free PS | −0.056 | .001 |
| Variable . | B . | P . |
|---|---|---|
| Sex | −0.412 | .216 |
| Age | 0.016 | .169 |
| FII | −0.205 | .860 |
| FV | −2.695 | .001 |
| Total PS | 0.005 | .690 |
| Free PS | −0.056 | .001 |
Multiple regression analysis, including sex (male = 0; female = 1), age, FII, FV, total PS, and free PS as independent variables, and the ETP-based nAPCsr as the dependent variable.
Neutrophil CD11b and plasma elastase
To evaluate the activation status of neutrophils, we measured both the levels of CD11b on the neutrophil surface and the plasma concentration of neutrophil elastase. Expression of CD11b on neutrophils was significantly elevated in both ET (144 ± 10.2 MFI; P < .001) and PV (158 ± 25 MFI; P = .007) patients compared with the control group (88 ± 6.5 MFI). No significant differences were found between JAK2V617F-positive and -negative patients.
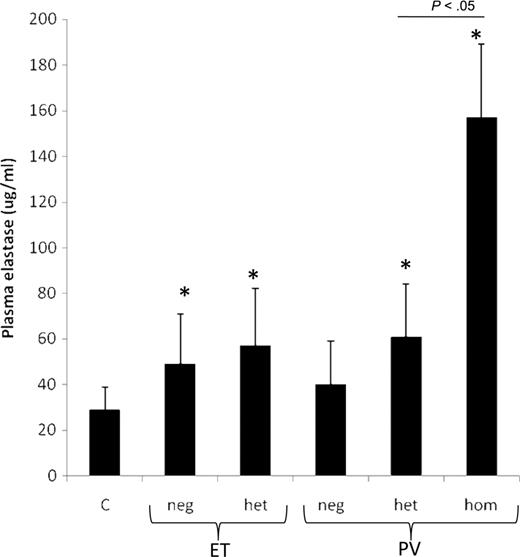
Figure 3 shows that elastase plasma concentrations were significantly higher in ET (53.2 ± 4.9 μg/L; P = .029) and PV patients (107 ± 25 μg/L; P = .024) than in healthy controls (29 ± 8.8 μg/L). The highest values were measured in the subgroup of PV subjects homozygous for the JAK2V617F mutation. Plasma neutrophil elastase was inversely correlated to FV (R = −0.268; P = .029), total PS (R = −0.461; P < .001), and free PS (R = −0.375; P = .003). The ratio between the plasma elastase concentration and the neutrophil count was calculated for each study subject. The mean values of this ratio remained significantly increased in ET (12.6 ± 1.3 μg/109 cells; P = .006) and PV patients (15.0 ± 3.6 μg/109 cells; P = .044) compared with controls (4.4 ± 1.28 μg/109 cells).
Plasma elastase levels in controls (C) and ET and PV patients. Plasma elastase levels (mean ± SE) are plotted according to disease type and to JAK2V617F mutational status. C indicates controls; Neg, negative; Het, heterozygous; and Hom, homozygous. *P < .01 versus controls.
Plasma elastase levels in controls (C) and ET and PV patients. Plasma elastase levels (mean ± SE) are plotted according to disease type and to JAK2V617F mutational status. C indicates controls; Neg, negative; Het, heterozygous; and Hom, homozygous. *P < .01 versus controls.
Discussion
The mechanisms underlying the hypercoagulable state and the increased thrombotic risk in patients with ET and PV are still unclear. One mechanism can be related to blood cell-induced activation of blood coagulation, which is more pronounced in JAK2V617F carrier subjects.15,18
The aims of this study were (1) to evaluate the overall coagulation phenotype of a group of patients with ET and PV using the thrombin generation assay and (2) to correlate this phenotype with JAK2V617F mutational status and leukocyte activation features. To detect possible abnormalities of the PS/PC system, this assay was performed in both the absence and the presence of APC.
Compared with healthy controls, ET and PV patients have lower ETP and peak height values of thrombin generation in the absence of APC but significantly higher ETP and peak height values in the presence of APC (Table 2), thus suggesting that their plasma is APC-resistant (Figure 1).
Plasma APC resistance is usually associated with carriership of the FV G1691A (FV Leiden) mutation. In line with previous studies, in which the FV Leiden mutation was not enriched in MPD patients,36-38 the APC-resistant phenotype of our ET and PV patients could not be explained by carriership of the FV Leiden mutation, which was present in only 3 of 89 patients (all belonging to the PV group).
This is the first study describing the occurrence of acquired APC resistance in ET and PV patients, as determined with the thrombin generation assay, whereas no plasma APC resistance could be detected with the aPTT-based APC resistance assay, in line with previous studies.36,39 This apparent discrepancy is because these 2 APC resistance assays explore different coagulation pathways and are sensitive to different plasma variables. The aPTT-based method is most sensitive to increased levels of FII and FVIII, and the ETP-based assay to decreased levels of free PS and TFPI.29,32 In particular, the aPTT test is not sensitive to PS levels in the 50% to 100% range.34
Because APC resistance, in the absence of FV Leiden, is also associated with an increased risk of thrombosis in other conditions, including pregnancy, oral contraceptive use, hormone replacement therapy, and cancer,31,35,40-42 acquired APC resistance in ET and PV patients may account for the thrombotic risk associated with these disorders.
It has been observed that, in MPD patients, a link exists between the JAK2V617F mutation burden and the activation state of both cell and plasma compartments of the hemostatic system.25,26 Particularly, JAK2V617F is associated with an increased expression of TF26 and P-selectin on platelet surface19,25 with increased platelet/neutrophil interactions,25,26 increased neutrophil expression of CD14 and leukocyte alkaline phosphatase,26,43 and plasma thrombomodulin levels.26 In addition, clinical data show an increased risk of thrombosis in JAK2V617F ET carriers.11-14 Based on this information, we analyzed the data from our ET and PV subjects according to the presence (ie, positivity or negativity) and the status (ie, heterozygosity or homozygosity) of the JAK2V617F mutation. This analysis indicated that JAK2V617F mutation carriers are more APC-resistant than noncarriers, especially if homozygous. Therefore, our data suggest a progression in the APC-resistant phenotype that is determined by the JAK2V617F status, and together with previous observations, supports the hypothesis of a more hypercoagulable condition in JAK2V617F carriers.
To understand the origin of the reduced sensitivity to APC in the thrombin generation assay, we measured the plasma levels of coagulation proteins known to be the major determinants of ETP-based APC resistance test,29,32 that is, FII, FV, total and free PS, and TFPI free. The results show a reduction in the levels of FV and free PS in patients compared with controls, and, mainly in JAK2V617F carriers. Interestingly, among JAK2V617F carriers, homozygous patients were characterized by significant lower levels of PS (total and free), FII and FV, compared with JAK2V617F heterozygous carriers. The multiple linear regression analysis indicated the low plasma levels of free PS and FV as the major determinants of the nAPCsr in our population. This is plausible as it has been demonstrated that small variations in the plasma concentration of free PS can cause significant variation in the ETP values determined in the presence of added APC.34 Reductions in the plasma levels of natural anticoagulants, particularly PS, were previously reported in patients with ET and PV.37-39,44 In our study, we not only confirmed these previous observations, but we could also demonstrate that the alterations in the coagulation protein levels induce an APC-resistant phenotype. At present, the causes of the reduced levels of FII, FV, and total and free PS in patients with ET and PV are unknown. One mechanism may be related to the ongoing chronic prothrombotic condition occurring in these diseases17 that causes a consumption of coagulation factors. The activation status of neutrophils frequently described in these subjects17,18,25,26 may determine both the hypercoagulable state and the decrease in the coagulation protein levels. Activated neutrophils interact intimately with hemostasis by regulating the activity of the coagulation cascade, through the release of proteolytic enzyme, such as elastase and cathepsin G.23 These enzymes have been implicated in the pathogenesis of some hemostatic disorders, including disseminated intravascular coagulation. Both PS (predominantly the free form) and FV can be degraded by elastase released from activated neutrophils.20,45,46 In all analyzed subjects in this study, the activation status of neutrophils was confirmed by the detection of increased CD11b expression on these cells as well as by increased plasma levels of elastase, particularly in JAK2V617F-positive patients. In addition, a significant inverse correlation was found between the plasma concentration of elastase and the levels of FV and total and free PS. An alternative mechanism can be that both neutrophils and platelets directly bind and degrade PS on their surface.47,48 In this case also, the increased number of platelets and leukocytes circulating in the blood of these patients might be responsible for the decreased concentration of PS and FV. Indeed, in our study subjects, a significant inverse correlation was observed between the platelet count and plasma level of FV, and between the leukocyte count and PS and FV. The 3 mechanisms are interrelated as the increased blood cell counts together with blood cell activation can contribute to the chronic activation of blood coagulation and hence to the consumption of coagulation factors.
Finally, acquired resistance to APC has been described in other types of cancer, including hematologic malignancies, such as multiple myeloma and lymphoma.41,42,49 However, in malignant conditions other than ET and PV, the appearance of APC-resistant phenotype has been determined by increased levels of plasma FV and factor VIII (FVIII), instead of decreased PS levels.40,41,50,51
In conclusion, we have shown that plasma from ET and especially PV patients is APC-resistant, which may account for the increased thrombotic risk associated with these disorders. This study provides a new insight in the pathogenesis of the thrombophilic state in patients with ET and PV by linking together the appearance of acquired APC resistance (a condition that is associated with an increased thrombotic risk), the occurrence of reduced levels of specific coagulation factors and neutrophil activation, and JAK2V617F mutational status. However, because these associations may not be causative, further studies on the relation between JAK2V617F mutation and coagulation alterations in MPD patients are warranted. In addition, because of the retrospective nature of this study, the predictive value of nAPCsr for thrombosis in the individual ET and PV patient could not be estimated. Therefore, prospective and longitudinal studies will be necessary to confirm our findings. The study suggests that targeting JAK2 molecular lesion may provide effective thromboprophylaxis to prevent thrombosis in MPD patients without anticoagulants.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants from the Annadal Foundation, Maastricht, The Netherlands (H.t.C.), the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy (A.F.), and the Dutch Organization for Scientific Research, The Netherlands (VIDI grant 917-76-312) (E.C.).
Authorship
Contribution: M.M. designed and performed research, analyzed data, and wrote the paper; E.C. performed research and analyzed data; H.M.H.S., R.v.O., and D.B. performed research; J.R. and T.B. contributed to the discussion; H.t.C. designed research; and A.F. designed research and contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Marchetti, Thrombosis and Hemostasis Center, Department of Hematology-Oncology, Ospedali Riuniti di Bergamo, Bergamo, Italy; e-mail: marina.r.marchetti@gmail.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal