Abstract
Neutralizing injurious stimuli, proinflammatory mediator catabolism, and polymorphonuclear leukocyte (PMN) clearance are determinants of inflammatory resolution. To this, we recently added innate-type lymphocyte repopulation as being central for restoring postinflammation tissue homeostasis with a role in controlling innate immune–mediated responses to secondary infection. However, although macrophages dominate resolution, their phenotype and role in restoring tissue physiology once inflammation abates are unknown. Therefore, we isolated macrophages from the resolving phase of acute inflammation and found that compared with classically activated proinflammatory M1 cells, resolution-phase macrophages (rMs) possess weaker bactericidal properties and express an alternatively activated phenotype but with elevated markers of M1 cells including inducible cyclooxygenase (COX 2) and nitric oxide synthase (iNOS). This phenotype is controlled by cAMP, which, when inhibited, transforms rM to M1 cells. Conversely, elevating cAMP in M1 cells transforms them to rMs, with implications for cAMP in the resolution of systemic inflammation. It transpires that although rMs are dispensable for clearing PMNs during self-limiting inflammation, they are essential for signaling postresolution lymphocyte repopulation via COX 2 lipids. Thus, rM macrophages are neither classically nor alternatively activated but a hybrid of both, with a role in mediating postresolution innate-lymphocyte repopulation and restoring tissue homeostasis.
Introduction
Macrophages possess a plasticity of phenotype that explains their protective as well as potentially detrimental role in chronic inflammation and tissue injury.1,2 Such heterogeneity arises as macrophages differentiate from monocytes and are exposed to specific tissue- and hematopoietic cell–derived stimuli. Macrophages are classified as either alternatively or classically activated. The “classical activation” (M1) profile occurs in a type 1 cytokine environment (IFNγ, TNFα) or upon recognition of pathogen-associated molecular patterns.3,4 Thus, M1 cells play an important role in protection against pathogens by producing high levels of TNFα, IL-1β, IL-6, and IL-12, for instance, and low levels of IL-10 and are therefore promoters of Th1-type immune responses.4 In addition, these cells express high levels of COX 2 and exert antiproliferative and cytotoxic activities resulting partly from reactive nitrogen and oxygen species including iNOS-derived NO, peroxynitrite, hydrogen peroxide, and superoxide.2 Although such short-term inflammatory activity is beneficial to the host in the terms of combating infection, the persistence of proinflammatory processes may results in tissue damage. Therefore, inflammation is counterbalanced by endogenous protective signals including Th2 cytokines (IL-10, IL-4, and IL-13) and apoptotic cells,5 resulting in the development of an anti-inflammatory or “alternatively activated” macrophage phenotype.6 Although exhibiting broad heterogeneity, such M2 cells express mannose receptors and secrete high levels of immunosuppressive IL-10 and TGFβ1 compared with M1 cells.2,6 .
Innate immune responses are characterized by the initial influx of polymorphonuclear leukocytes (PMNs) followed by monocyte-derived macrophages, leading to resolution followed by injured tissues returning to normal physiology.7-10 Although a great deal is known about the properties of early release cytokines and chemokines and their mechanisms of action that drive inflammation, we are still trying to understand more about the soluble mediators and cellular players that trigger resolution and restoration of tissue homeostasis. To this end, we recently identified innate-type lymphocyte repopulation as being central to restoring tissue normal physiology once inflammation resolves, with a role in controlling responses to secondary infection.11 However, given the predominance of macrophages during resolution, the phenotype of these cells is unknown. Therefore, we set about cataloging the inflammatory status of resolution-phase macrophages and documenting their role in postinflammation tissue homeostasis.
Therefore, a model of self-limiting peritonitis was established from which macrophages could be isolated from its resolution phase. A model of peritonitis that progressed to systemic inflammation was also established for comparison. Using cytokine arrays and proteomic analysis, we confirmed that the inflammatory environment macrophages occupied during resolution was anti-inflammatory, whereas the cavity of animals that progressed to systemic inflammation expressed proinflammatory cytokines and acute-phase proteins. Macrophage phenotyping revealed that whereas cells from the nonresolving model were M1, resolution-phase macrophages (rMs) were alternatively activated but also expressed high levels of COX 2 as well as iNOS, imparting upon them a hybrid phenotype. These macrophages also differed in their abilities to clear bacterial infection, with M1 cells being superior to rM cells in this regard. The phenotype of macrophage populations was determined by intracellular cAMP, which is elevated in rMs and if inhibited transforms their phenotype to that of M1 cells. It transpires that although rMs are dispensable for PMN clearance, they signal the repopulation of innate-type lymphocytes, one of the critical determinants of successful resolution and tissue homeostasis.11
Methods
Animal maintenance and induction of peritonitis
All animals (C57BL6/J) were bred under standard conditions and maintained in a 12-hour light/12-hour dark cycle at 22°C plus or minus 1°C and given food and tap water ad libitum in accordance with United Kingdom Home Office regulations. Peritonitis was induced by the intraperitoneal injection of either 4 or 400 mg/kg type A zymosan (Sigma-Aldrich, Poole, United Kingdom), and cells were enumerated by hemocytometer at time points stated in “Results” by sterile PBS washout. For pharmacological experiments, 200 μL 1 mM rp-adenosine 3′,5′-cyclic monophosphorothioate (rp-cAMP; Sigma-Aldrich) was injected intraperitoneally 24 and 16 hours prior to isolation of macrophages. Rolipram (30 mg/kg) and theophylline (100 mg/kg) were given orally from 24 hours. Clodronate (100 μL intravenously), encapsulated in liposomes as described earlier,12 was a gift of Roche Diagnostics (Mannheim, Germany). This study received institutional review board approval for the use of mice from the United Kingdom Home Office.
Two-dimensional electrophoresis
Cell-free peritoneal exudates (1 mL) were concentrated using Centricon YM-10 spin column (Millipore, Billerica, MA). Samples were centrifuged at 5000g for 2 hours to obtain a 20-fold concentration. Concentrated exudates were used for either Western blotting or 2D gel electrophoresis. For 2D gel electrophoresis, albumin was removed using an albumin removal column (Calbiochem, San Diego, CA) and samples were concentrated using Centricon YM-10 spin column with protein concentrations determined by Bradford assay (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom). Protein (50 μg) was resuspended in 2D buffer (7 M urea, 2 M thiourea, 4% wt/vol CHAPS) and subjected to isoelectric focusing on 7-cm acrylamide strips, nonlinear pH gradient 3 to 10 using an IPGphor (GE Healthcare, Little Chalfont, United Kingdom). Equilibration of the strips to the SDS buffer system (6 M urea, 2% wt/vol SDS, 30% vol/vol glycerol, and 0.002% wt/vol bromophenol blue in 50 mM Tris-HCl buffer; pH 8.8) was performed in 2 15-minute incubations: the first in SDS buffer containing 1% wt/vol DTT, the second in SDS buffer containing 2.5% wt/vol iodoacetamide. Electrophoresis in the second dimension was performed after applying the strips to 10% acrylamide gels.
Silver staining and mass spectrometry
One- and 2-dimensional gels were fixed by overnight incubation in 40% vol/vol ethanol/10% vol/vol acetic acid and silver stained using a mass spectrometric compatible protocol.13 Spots/bands of interest were excised from the gel and underwent reduction by DTT, followed by alkylation with iodoacetamide and dehydration in acetonitrile. The dehydrated gel plugs were rehydrated with 20 ng/μL trypsin (Trypsin Gold; Promega, Southampton, United Kingdom) and incubated overnight at 37°C in a humid chamber. Trypsin digests were spotted onto a stainless steel target together with matrix (a saturated solution [∼10 mg/mL] of α-cyano-4-hydroxycinnamic acid diluted 1:2 in 33% vol/vol acetonitrile and 0.1% vol/vol trifluoroacetic acid) and peptide masses were determined by matrix assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Mass Spectrometer (Bruker Autoflex, Bruker Daltonics, United Kingdom). Proteins were identified by submitting the peptide masses to the Mascot online database (http://www.matrixscience.com).14
Western blotting and FACS analysis
Western blotting was carried as described previously.15 For HMGB1 detection, antibodies and blocking peptides for characterization were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescence-activated cell sorting (FACS) was carried out on Becton Dickinson FacsCalibur with data analyzed by Cellquest (San Jose, CA). Leukocytes were incubated with antibodies to Gr1 (Ly6C/LY6G), Ly6C, F4/80 (Caltag Laboratories, Burlingame, CA), Ly6G, CD11B, CD11C (both BD Biosciences, San Jose, CA), or CD3/CD19 (AbD Serotec, Raleigh, NC) using isotype-specific antibodies from each respective supplier and compensated where appropriate for dual labeling. Detection of mannose receptor was carried out after cells were permeabilized per manufacturer's instructions using Leukoperm, anti-CD206 antibody, and isotype control from AbD Serotec. For apoptosis analysis, cells were incubated with ANX V/PI (BD Biosciences).
Cytokine, chemokine, proliferation, and cAMP analysis
Cytokines and chemokines were measured by Multiplex cytokine array analysis (Bio-Rad Laboratories) using the manufacturer's protocols. cAMP was measured by enzyme immunoassay (Cayman Chemicals, Ann Arbor, MI). To determine whether rM or M1 cells proliferated, BrdU (BD Biosciences) was injected intraperitoneally to mice bearing resolving or nonresolving inflammation 24 hours after zymosan injection. Three hours after BrdU, rM and M1 cells were isolated and BrdU incorporation was determined on fixed cells by FACS analysis counterstaining with F4/80 to identify macrophage populations using large granular macrophage in the naive peritoneum as a positive control.
Eicosanoid analysis
Samples stored at −200°C were thawed at room temperature and acidified to pH 3. Solid-phase extraction was performed using C18 cartridges and samples were analyzed for levels of PGD2 using enzyme immunoassay (Cayman Chemicals).
Bacterial culturing
Staphylococcus aureus (serotype V) was grown in Luria broth and group B streptococcus was grown in Bacto Todd Hewitt broth with agitation at 37°C to an OD600 of 0.4; equivalent to 1 × 108 cfu/mL. Bacteria collected by centrifugation were washed with sterile PBS. For in vivo experiments, mice were inoculated intraperitoneally with 3 × 107 cfu group B streptococcus in 300 μL PBS. For in vitro experiments, 0.2 × 106 cfu/mL of unwashed S aureus was used.
In vitro cell stimulation and bacterial killing assay
Peritoneal cells were resuspended in Dulbecco modified medium supplemented with 10% fetal bovine serum and 50 μg/mL penicillin and streptomycin, and 0.5 × 106 cells per well were seeded in a 48-well plate and left to adhere for 1 hour in a humidified CO2 incubator. After washes, fresh medium was added and the cells were incubated for either 4 or 24 hours. In some experiments, LPS (Sigma-Aldrich), S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]–cycteinyl-[S]seril-3[S]-lysyl-[S]-lysine (PAM-CYS), polyinosine-polycytidylic acid (poly I:C), or muramyl dipeptide (MDP) (InvivoGen, San Diego, CA) was added to the wells prior to a 20-hour incubation. In other experiments, N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (db-cAMP) or rp-adenosine 3′,5′-cyclic monophosphorothioate (rp-cAMP) (Sigma-Aldrich) was added to the wells with or without addition of LPS prior to a 4-hour incubation. At the end of the incubation, cell-free supernatants were removed and stored in −80°C until further analysis. To assess macrophage bacterial killing ability, 0.2 × 106 cfu/mL S aureus was added to appropriate wells. Plates were centrifuged for 5 minutes at 400g and incubated for 1 hour. Subsequent supernatants were subjected to X4 10-fold dilutions in water prior to dispensing 100 μL of the dilutions on to agar plates. The plates were incubated upside down at 37°C, and bacterial colonies were counted 24 hours later. In some experiments, preconditioned macrophages from mice injected with rp-cAMP 24 and 16 hours prior to the experiment were used, and, further, more adhered cells were preincubated with rp-cAMP (preconditioned cells) or db-cAMP (nontreated cells) for 4 hours prior to addition of S aureus.
Macrophage labeling and adoptive transfer
For trafficking experiments, the macrophage-specific stain PKH26-PCLred or PKH2-PCLgreen (Sigma-Aldrich; 2 mL of 500 nM) was injected into the inflamed peritoneal cavity of mice at time points indicated in “Results” with cells isolated at 72 hours. Using FACS, cells staining positively for PKH26-PCLred (FL2 channel) were identified with FITC-labeled F4/80 (FL1 channel), whereas those positive for PKH2-PCLgreen were identified by PE-labeled F4/80 (FL-2 channel). For rM and M1 clearance experiments, MΦs were labeled with either PKH26-PCLred or PKH2-PCLgreen and mixed in equivalent proportions, and 4 × 106 cells were injected intraperitoneally with the remaining numbers determined by FACS 24 hours later. For adoptive transfer experiments, animals bearing a 0.1 mg zymosan-induced peritonitis and containing rM cells were injected with PKH26-PCL intraperitoneally at 72 hours. Three hours later, rMs were isolated and 4 × 106 injected into mice bearing ongoing inflammation triggered by 10 mg zymosan at 72 hours, and the effects of rMs on cell numbers were determined 24 hours later. To characterize the phenotype of these adoptively transferred rMs at 96 hours in the 10-mg zymosan model, 50 × 103 rMs from each animal were sorted by FACSAria (BD, Franklin Lakes, NJ) and incubated as before for 4 hours in culture to determine cytokine secretion.
Results
Establishing a model of resolving versus delayed-resolving inflammation
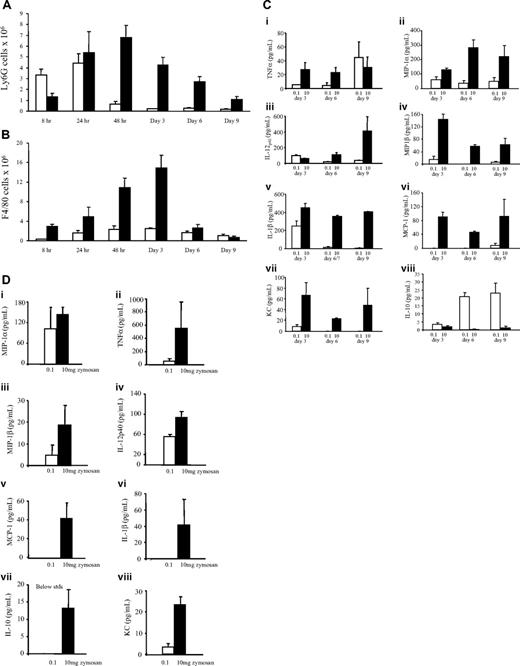
We established a model of resolving peritonitis as well as a more severe model for comparison that progresses to systemic inflammation by injecting 2 distinct quantities of zymosan intraperitoneally into 2 separate groups of mice. Thus, a low dose of 0.1 mg, which triggers a mild and transient inflammation resulting in full recovery, and a higher dose of 10 mg, which results in a more progressive and prolonged response leading to systemic inflammation, were used. The latter dose visibly affected mice, which exhibited hunching, weight loss, and in some cases multiple organ failure consistent with previous publications.16,17 Cells were isolated from the inflamed peritoneum of both models and quantified with differential cell counts determined by FACS analysis throughout the response. In low-dose zymosan, PMNs peaked at 8 or 24 hours and cleared thereafter, with macrophages peaking at 72 hours and persisting in the peritoneum for up to 3 weeks after stimulation (Figure 1A,B). By contrast, in response to high-dose zymosan total PMN and macrophages were considerably higher than in the resolving model peaking at 72 hours (Figure 1A,B). Analysis of inflammatory exudates revealed elevated levels of proinflammatory cytokines and chemokines in exudates of mice that received 10 mg zymosan up to day 9 (Figure 1C) and in their plasma at day 16 (Figure 1D), indicative of systemic inflammation. In contrast, cell-free inflammatory exudates of resolving inflammation contained predominantly IL-10 and comparatively little proinflammatory mediators in their plasma (Figure 1C and D, respectively). Thus, by injecting different levels of an inflammatory irritant, we established a model of resolving peritonitis as well as one that progressed to systemic inflammation. As a result, we propose that this self-limiting model (induced by 0.1 mg zymosan) be used to identify novel endogenous resolution pathways that may be dysregulated or absent in the delayed or nonresolving inflammation.
Cell profile and associated cytokine and chemokine release in resolving inflammation. We established 2 models of inflammation—one where a low dose of zymosan (0.1 mg, □) was injected intraperitoneally causing a transient acute inflammatory response that resolved, as well as another model where 10 mg zymosan was injected (■) triggering a more aggressive inflammation. (A) Gr1(LY6G)-positive granulocytes and (B) F4/80-labeled macrophages were enumerated in each model over time by hemocytometer and FACS analysis. (C) Levels of typical proinflammatory cytokines were quantified in the cell-free inflammatory exudates at several time points during and after resolution in both models as well as (D) in plasma 2 weeks after resolution to determine whether inflammation resolved or became systemic. n = 6 to 8 mice per group with data expressed as mean plus or minus SEM.
Cell profile and associated cytokine and chemokine release in resolving inflammation. We established 2 models of inflammation—one where a low dose of zymosan (0.1 mg, □) was injected intraperitoneally causing a transient acute inflammatory response that resolved, as well as another model where 10 mg zymosan was injected (■) triggering a more aggressive inflammation. (A) Gr1(LY6G)-positive granulocytes and (B) F4/80-labeled macrophages were enumerated in each model over time by hemocytometer and FACS analysis. (C) Levels of typical proinflammatory cytokines were quantified in the cell-free inflammatory exudates at several time points during and after resolution in both models as well as (D) in plasma 2 weeks after resolution to determine whether inflammation resolved or became systemic. n = 6 to 8 mice per group with data expressed as mean plus or minus SEM.
Dynamics of monocyte/macrophage trafficking in resolving inflammation
To determine macrophage trafficking in resolving inflammation, we characterized the influx of monocytes in response to zymosan (0.1 compared with 10 mg) and their cell surface markers as being Ly6Chi and F4/80− in both models. These monocytes differentiated into macrophages (Ly6Clo and F4/80hi) from 24 hours onward, becoming progressively larger and more granular over time with the degree of differentiation correlating with increased F4/80 expression and reduced Ly6C labeling (Figure S1A,B, available on the Blood website; see the Supplemental Figures link at the top of the online article). We next injected the phagocyte-specific label PKH26-PCLred intraperitoneally to both models at 24 hours and analyzed samples at 72 hours, the phase of peak macrophage occupancy in these models (Figure 1B). PKH26-PCLred has been shown by others to be rapidly phagocytosed and cleared from the inflammatory environment within minutes of injection.18,19 In confirmatory experiments, we found that PKH26-PCL is cleared within 1 hour by incubating cell-free inflammatory exudates from animals injected with PKH26-PCL with macrophages in vitro and finding that cultured macrophages did not label positively with PKH26-PCL (data not included). Therefore, this method of cell labeling provides a valuable tool with which to determine macrophage trafficking in vivo. The results of these experiments revealed that in resolving inflammation virtually all macrophages stained positively for PKH26-PCLred, whereas in the 10-mg zymosan group about half were PKH26-PCLred (Figure S1C,D). Taking this further, 12 hours after the initial PKH26-PCLred injection animals were also injected with PKH2-PCLgreen intraperitoneally and cells analyzed at 72 hours, the idea being that subsequent influxing macrophages would label positively with PKH2-PCLgreen only. These results confirm that in resolving inflammation very few macrophages were green only, whereas in nonresolving inflammation about 15% were PKH2-PCLgreen positive (Figure S1E,F). Therefore, in resolving inflammation rMs are derived from a common monocyte precursor that had migrated into the peritoneum by 24 hours, whereas in nonresolving inflammation there is a more prolonged influx of monocytes over a broader time frame than when inflammation is self-limiting.
Distinct macrophage phenotype in a proresolution environment
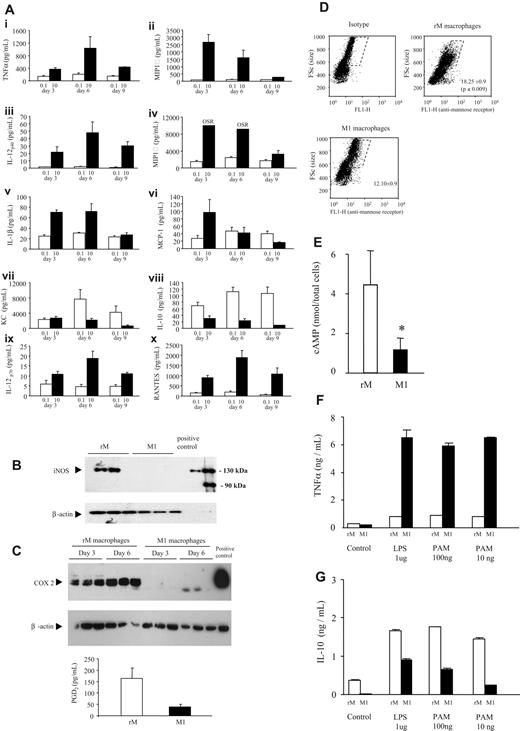
Using these models of resolving and nonresolving inflammation, we sought to establish the phenotypes of macrophages taken from a resolving inflammation (0.1 mg zymosan) compared with those taken from a nonresolving or proinflammatory environment (10 mg zymosan). To this end, macrophages from 72 hours were isolated and incubated for 6 hours ex vivo to determine the profile of cytokines and chemokines secreted as well as intracellular proteins they express. As expected, macrophages from 10 mg zymosan produced high levels of TNFα, IL-12p40, as well as MIP-1α and MIP-1β, for instance, along with lower levels of IL-10 (Figure 2A). Because of their similarity to classically activate macrophages, these cells will be referred to as M1. On the other hand, macrophages from the resolving inflammatory environment synthesized high levels of anti-inflammatory IL-10 (Figure 2A) as well as iNOS (Figure 2B), COX 2, the COX 2–derived anti-inflammatory lipid mediator PGD2 (Figure 2C), mannose receptor (Figure 2D), along with comparatively higher levels of cAMP (Figure 2E). These macrophages produced little of the proinflammatory cytokines or chemokines typical of the 10-mg zymosan model. These data suggest that resolving inflammation favors a macrophage phenotype that is neither classically nor alternatively activated but a hybrid of both canonical definitions, referred to as resolving-phase macrophages (rMs). Macrophages were then stimulated ex vivo for 6 hours with LPS (TLR-4), PamCys (TLR-2), or poly I:C (TLR-3). Using TNFα and IL-10 as surrogate determinants of inflammatory status, we found that M1 cells consistently elaborated preferentially higher levels of TNFα (Figure 2F), whereas rM cells synthesized more IL-10 (Figure 2G). These experiments demonstrated the ex vivo robustness of these contrasting macrophage phenotypes in response to a range of defined inflammatory stimuli.
Unique phenotype of resolution-phase macrophages is preserved in response to inflammatory stimuli ex vivo. Macrophages were isolated from a 72-hour peritonitis that either resolved (0.1 mg zymosan or rM macrophages, □) or progressed to systemic inflammation (10 mg zymosan or M1 macrophages, ■) and incubated for 6 hours ex vivo to determine profiles and levels of (A) cytokines and chemokines indicative of established M1 and M2 macrophage phenotype. Having established that rM macrophages secrete predominantly anti-inflammatory IL-10 and comparatively fewer proinflammatory mediators, expression of other inflammatory markers was determined including (B) iNOS as well as (C) COX 2 and COX 2–derived PGD2 and intracellular markers of M2 phenotype including (D) mannose receptor as well as (E) cAMP. (F,G) Finally, we determined that exposure of these resolution-phase macrophages to a range of inflammatory stimuli did not alter their phenotype, which remained robust upon exposure to TLR ligands. n = 6-8 mice per group. *P ≤ .05, as determined by ANOVA, followed by Bonferroni t test or 2-tailed Student t test, with data expressed as mean plus or minus SEM.
Unique phenotype of resolution-phase macrophages is preserved in response to inflammatory stimuli ex vivo. Macrophages were isolated from a 72-hour peritonitis that either resolved (0.1 mg zymosan or rM macrophages, □) or progressed to systemic inflammation (10 mg zymosan or M1 macrophages, ■) and incubated for 6 hours ex vivo to determine profiles and levels of (A) cytokines and chemokines indicative of established M1 and M2 macrophage phenotype. Having established that rM macrophages secrete predominantly anti-inflammatory IL-10 and comparatively fewer proinflammatory mediators, expression of other inflammatory markers was determined including (B) iNOS as well as (C) COX 2 and COX 2–derived PGD2 and intracellular markers of M2 phenotype including (D) mannose receptor as well as (E) cAMP. (F,G) Finally, we determined that exposure of these resolution-phase macrophages to a range of inflammatory stimuli did not alter their phenotype, which remained robust upon exposure to TLR ligands. n = 6-8 mice per group. *P ≤ .05, as determined by ANOVA, followed by Bonferroni t test or 2-tailed Student t test, with data expressed as mean plus or minus SEM.
cAMP determines the phenotype of rM cells
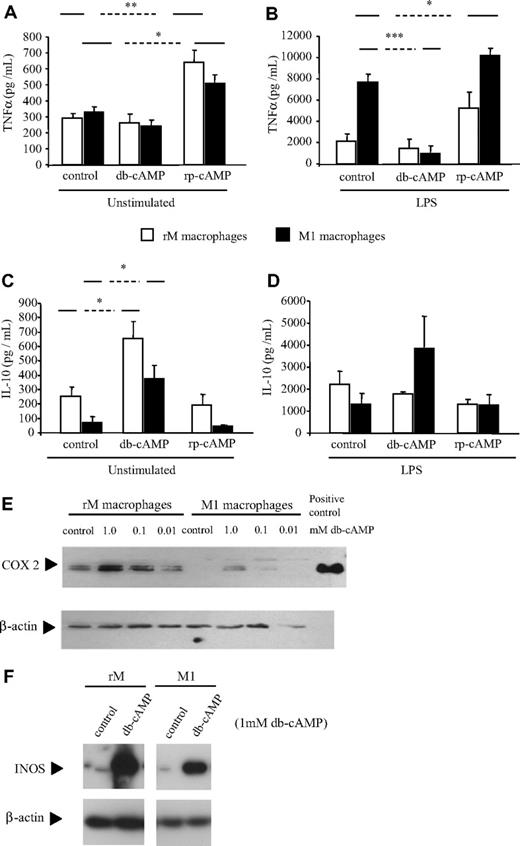
Given the differential expression of cAMP in resolving versus nonresolving macrophages, we examined whether macrophage phenotype is dictated by cAMP and whether this phenotype could be altered by changing intracellular levels of this potent intracellular second messenger.20 To this end, we used the cAMP analog, dibutyryl (db) cAMP,21,22 and the cAMP antagonist, rp-cAMP,23 which, like native cAMP binds protein kinase A, but without activating the enzyme. Thus, macrophage response to cAMP modulation was determined by measuring TNFα and IL-10 secretion as well as COX 2 and iNOS expression as markers of phenotypes specific to each respective macrophage subtype. Both cell types showed an increase in IL-10 production as well as COX 2 and iNOS expression in response to db-cAMP and an increase in TNFα with rp-cAMP (Figure 3A-F). In M1 cells, TNFα production was attenuated by db-cAMP, whereas IL-10 production was increased, suggesting a reversion toward the anti-inflammatory or rM phenotype.
Resolution-phase macrophage phenotype is determined by cAMP. Macrophages (0.5 × 106) previously classified as either resolution phase (rM) or M1 were treated with modulators of cAMP with or without LPS for 20 hours in triplicate. Using (A,B) TNFα and (C,D) IL-10 as markers of macrophage inflammatory status, it was determined that cAMP elevation in M1 cells triggered IL-10 while also inhibiting TNFα. Changes in (E) COX 2 and (F) iNOS expression in response to cAMP were determined by Western blot analysis using 3 μg M1 and rM cell lysates in each lane. LPS-stimulated J774 macrophage extracts were used as positive controls. n = 6 to 8 mice per group. *P ≤ .05, as determined by ANOVA, followed by Bonferroni t test with data expressed as mean plus or minus SEM.
Resolution-phase macrophage phenotype is determined by cAMP. Macrophages (0.5 × 106) previously classified as either resolution phase (rM) or M1 were treated with modulators of cAMP with or without LPS for 20 hours in triplicate. Using (A,B) TNFα and (C,D) IL-10 as markers of macrophage inflammatory status, it was determined that cAMP elevation in M1 cells triggered IL-10 while also inhibiting TNFα. Changes in (E) COX 2 and (F) iNOS expression in response to cAMP were determined by Western blot analysis using 3 μg M1 and rM cell lysates in each lane. LPS-stimulated J774 macrophage extracts were used as positive controls. n = 6 to 8 mice per group. *P ≤ .05, as determined by ANOVA, followed by Bonferroni t test with data expressed as mean plus or minus SEM.
Compared with M1, rM cells have reduced bactericidal properties
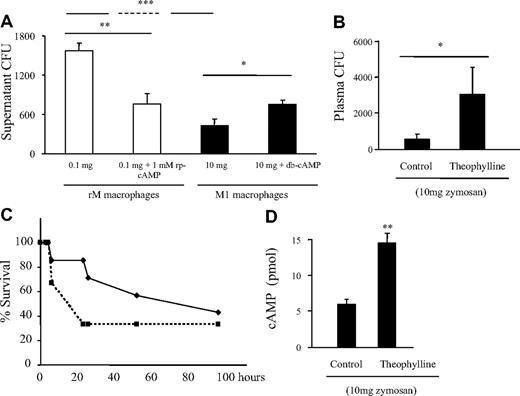
The role of macrophages during bacterial infections is well documented, as is the inhibitory effect that IL-10 has on macrophage bacterial clearance.24-28 In agreement with this established paradigm, we found that M1 cells from the proinflammatory model (10 mg zymosan) were more efficient at clearing S aureus than rM cells (Figure 4A). More importantly, rM's ability to kill bacteria was enhanced by incubation with rp-cAMP (cAMP inhibitor) prior to addition of bacteria. Conversely, the killing ability of proinflammatory macrophages (M1) was reduced by addition of db-cAMP. These findings suggest that modulating macrophage phenotype may alter host ability to combat bacterial infection. To test this hypothesis in vivo, we injected the phosphodiesterase IV inhibitor theophylline at concentrations that elevate cAMP at 72 hours to M1-sufficent, proinflammatory mice (10 mg zymosan). Thirty minutes after theophylline, group B streptococcus was injected intraperitoneally and plasma was taken 3 hours later to determine bacterial load. Theophylline caused an increase in plasma bacterial counts compared with controls and was associated with increased mortality (Figure 4B,C), showing that converting M1 macrophages to bactericidal-impaired rMs allows for enhanced bacterial colonization and mortality concomitant with elevated cAMP (Figure 4D). Equivalent data were obtained with rolipram. The results of these experiments suggest that the phenotypic status of inflammatory macrophages may be a critical determinant of host ability to combat postinflammation superinfection and that drugs that elevate cAMP may compromise ability of the innate immune response to kill bacteria.
Resolution-phase macrophages possess reduced bactericidal properties compared with M1 cells. (A) Macrophages (0.5 × 106) were isolated from a 72-hour peritonitis that either resolved (0.1 mg zymosan or rM macrophages, □) or progressed to systemic inflammation (10 mg zymosan or M1 macrophages, ■) and incubated with S aureus. One hour later, media were taken and plated on LB-agar plates and bacterial colonies counted 24 hours later as a measure of macrophage ability to kill bacteria. Thus, the higher CFU reflect reduced bacterial clearance. In the first instance, macrophages from resolving inflammation (rM) had a lower ability to kill S aureus compared with M1 cells. However, upon incubation with the cAMP inhibitor, rp-cAMP, rM cells experienced enhanced bactericidal properties. Conversely, M1 cells, whose inherent ability to kill bacterial was greater than that of rM cells, were reduced by elevating cAMP. (B) Theophylline was injected to animals bearing an ongoing inflammation (10 mg zymosan) at 72 hours followed by group B streptococcus with plasma sampled 3 hours later to determine colony-forming units along with (C) animal survival over time at concentrations of drug that (D) elevated cAMP. n = 8 mice per group. *P ≤ .05; **P ≤ .01; and ***P ≤ .001, as determined by ANOVA, followed by Bonferroni t test with data expressed as mean plus or minus SEM.
Resolution-phase macrophages possess reduced bactericidal properties compared with M1 cells. (A) Macrophages (0.5 × 106) were isolated from a 72-hour peritonitis that either resolved (0.1 mg zymosan or rM macrophages, □) or progressed to systemic inflammation (10 mg zymosan or M1 macrophages, ■) and incubated with S aureus. One hour later, media were taken and plated on LB-agar plates and bacterial colonies counted 24 hours later as a measure of macrophage ability to kill bacteria. Thus, the higher CFU reflect reduced bacterial clearance. In the first instance, macrophages from resolving inflammation (rM) had a lower ability to kill S aureus compared with M1 cells. However, upon incubation with the cAMP inhibitor, rp-cAMP, rM cells experienced enhanced bactericidal properties. Conversely, M1 cells, whose inherent ability to kill bacterial was greater than that of rM cells, were reduced by elevating cAMP. (B) Theophylline was injected to animals bearing an ongoing inflammation (10 mg zymosan) at 72 hours followed by group B streptococcus with plasma sampled 3 hours later to determine colony-forming units along with (C) animal survival over time at concentrations of drug that (D) elevated cAMP. n = 8 mice per group. *P ≤ .05; **P ≤ .01; and ***P ≤ .001, as determined by ANOVA, followed by Bonferroni t test with data expressed as mean plus or minus SEM.
Inflammatory environment dictates macrophage phenotype
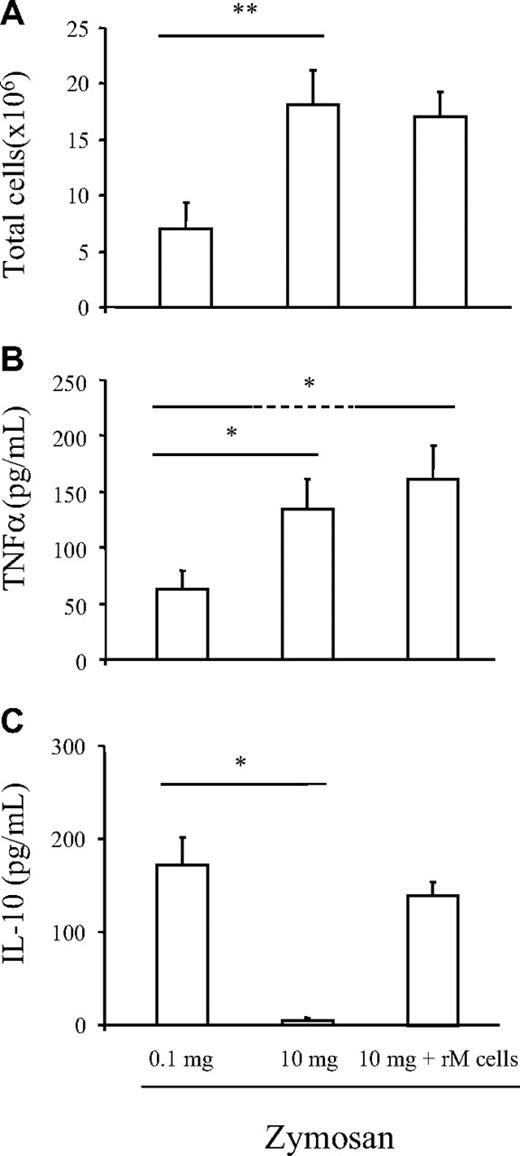
We next questioned whether rM cells could alter the course of ongoing inflammation. rMs were labeled with PKH26-PCLred and adoptively transferred at 72 hours to mice bearing a 10 mg zymosan-induced (nonresolving) peritonitis, with their impact on inflammation determined 24 hours later. However, rM cells did not trigger resolution in these animals (Figure 5A). Specifically, there was no reduction in total PMNs or macrophages or alterations in lymphocytes numbers (individual cell profile not included). Lymphocytes are pertinent markers of resolution, as we have shown recently that lymphocyte repopulation is critical for postresolution tissue homeostasis.11 As one explanation for this negative result, transferred PKH26-PCLred–labeled rMs, when separated from the nonresolving milieu after 24 hours using FACSAria, were found to synthesize more TNFα than native rM cells with little difference in IL-10 (Figure 5B,C). This indicates that despite the robustness of ex vivo stimulation in response to a defined stimulus as shown in Figure 2F,G, the environment of nonresolving inflammation predominated in vivo, transforming rMs toward an M1 phenotype. Similar results were obtained when rolipram and theophylline were administered to mice bearing 10 mg zymosan where cAMP reduced M1 TNFα but had no effect on leukocyte numbers (Figure S2E,F). Thus, although elevating cAMP dampens proinflammatory cytokines with implications for resolution of systemic inflammation, it is insufficient to reduce leukocyte numbers in the peritoneum. We suspect that endogenous mediators, specific to ongoing inflammation, prevail over proresolution signals in terms of regulating cell clearance. Proteomic analysis shed some light on these respective environments, finding several acute-phase proteins including haptoglobin, hemopexin, pregnancy zone protein, and serum amyloid P-component as well as proinflammatory HMGB-1 expressed during ongoing inflammation (Figure S2A-D).29 In contrast, proteins from resolving inflammation were largely anti-inflammatory, defined by markers of alternatively activated macrophages (arginase I)30 and proteins involved in antioxidative pathways mediated by glutathione (carbonic anhydrase 3)31 and interestingly proteins involved in energy metabolism (triosephosphate isomerase,32,33 creatine kinase,34 and sorbitol dehydrogenase35 ). Thus, although we can alter the phenotype of macrophages with implications for resolution of systemic inflammation, endogenous proinflammatory signals of the type identified by proteomic analysis may control the fate of leukocytes in a manner that is independent of cAMP. In which case, we propose that cAMP is elevated as a consequence of resolution once signals that drive inflammation are dampened and is not required for leukocyte clearance.
Inflammatory environment dictates macrophage phenotype. rM cells from a 72-hour zymosan (0.1 mg)–induced peritonitis were labeled with PKH26-PCLred and adoptively transferred at 72 hours to mice bearing a 10 mg zymosan (nonresolving inflammation) peritonitis and found not to trigger resolution as defined by little alterations in (A) total as well as individual cell numbers (PMNs, MΦs, and innate-type lymphocytes, not shown) determined 24 hours later. However, adoptively transferred rMs (identified by being PKH26-PCLred) were isolated from the nonresolving milieu after 24 hours using FACSAria and were found to synthesize more (B) TNFα than native rM cells with little change in (C) IL-10, indicating that despite the robustness of ex vivo stimulation reported in Figure 2F,G, the inflammatory environment of whole animal systems dictates cellular phenotypes. n = 6 mice per group. * indicates P ≤ .05, and **P ≤ .01, as determined by ANOVA, followed by Bonferroni t test with data expressed as mean plus or minus SEM.
Inflammatory environment dictates macrophage phenotype. rM cells from a 72-hour zymosan (0.1 mg)–induced peritonitis were labeled with PKH26-PCLred and adoptively transferred at 72 hours to mice bearing a 10 mg zymosan (nonresolving inflammation) peritonitis and found not to trigger resolution as defined by little alterations in (A) total as well as individual cell numbers (PMNs, MΦs, and innate-type lymphocytes, not shown) determined 24 hours later. However, adoptively transferred rMs (identified by being PKH26-PCLred) were isolated from the nonresolving milieu after 24 hours using FACSAria and were found to synthesize more (B) TNFα than native rM cells with little change in (C) IL-10, indicating that despite the robustness of ex vivo stimulation reported in Figure 2F,G, the inflammatory environment of whole animal systems dictates cellular phenotypes. n = 6 mice per group. * indicates P ≤ .05, and **P ≤ .01, as determined by ANOVA, followed by Bonferroni t test with data expressed as mean plus or minus SEM.
rM mediates postresolution homeostasis
To gain further insight into the role rM cells play during resolution, macrophages were depleted using clodronate-filled liposomes in animals injected with 0.1 mg zymosan intraperitoneally. Despite macrophages being expectedly reduced by 85% at resolution following clodronate treatment (Figure 6B), PMN numbers were also significantly lower in clodronate-treated animals (Figure 6C). This was a particularly striking result given that PMN influx in macrophage-depleted animals was significantly higher than in controls at onset (4 hours, Figure 6A), suggesting that in the presence of limited phagocytosing macrophage numbers, PMNs may be efficiently cleared by stromal cells and/or via lymphatic drainage at resolution. In a recent report, we demonstrated the influx of innate-type lymphocytes during inflammatory resolution in rodents and humans.11 These repopulating lymphocytes had no role in actively switching off inflammation (clearing PMNs and/or macrophages) but restored tissue homeostasis and controlled against superinfection. In macrophage-depleted animals examined here, we noted significantly fewer repopulating lymphocytes compared with controls at resolution (Figure 6D), indicating that one of the functions of rMs during resolution is to trigger lymphocyte repopulation and restore normal physiological function after inflammation. Taking this further, we found that rM COX 2 liberated an arachidonic acid–derived prostanoid that signaled lymphocyte repopulation as administration of a selective COX 2 inhibitor during resolution impaired CD3 cell repopulation (Figure 6E). Moreover, adoptively transferring PKH26-PCLred–labeled rM along with equal numbers of PKH2-PCLgreen–labeled M1 cells to a murine peritoneal cavity revealed that after 24 hours rM cells remained in the peritoneum, whereas M1 cells displayed a greater clearance rate (Figure 6F,G). Given that neither rM nor M1 cells were found to proliferate as determined by BrdU incorporation (data not included), these data suggest that rM cells possess a greater propensity to remain in the peritoneum to elicit prohomeostatic functions via COX 2–derived lipid mediators.
rM mediates postresolution homeostasis. Mice were injected intravenously with clodronate-filled liposomes followed by zymosan (0.1 mg). Inflammation was first determined 4 hours later showing a significant increase over controls of (A) PMNs at onset followed by a confirmed reduction in (B) macrophages during resolution (48 hours) concomitant with a surprising reduction in (C) PMN numbers during this phase, suggesting that when phagocytosing macrophage are limited in numbers, PMNs may be efficiently cleared by parenchymal/stromal cells. However, (D) T- and B-lymphocyte numbers were also significantly reduced, revealing that rM cells are central to recruiting postresolution innate-type lymphocytes, which we have shown to be critical for postinflammation susceptibility to superinfection and restoration of tissue homeostasis.11 (E) We identified that an rM COX 2–derived prostanoid mediated these effects as NS-398 at 10 mg/kg dosed orally during resolution (48 and 60 hours) impaired CD3 cell repopulation at 72 hours. Moreover, (F) adoptively transferring PKH26-PCLred rM along with equal numbers of PKH2-PCLgreen M1 cells to a murine peritoneal cavity revealed that after 24 hours rM cells remained in the peritoneum, whereas M1 displayed a greater clearance rate, suggesting that rM cells possess a greater propensity to remain in the peritoneum to elicit their prohomeostatic functions, with the dotted line denoting the original numbers of MΦs injected. (G) FACS dot plot to prove the successful transfer of differentially labeled MΦs and illustrating the greater number of PKH26-PCLred MΦs (bottom right quadrant) compared with fewer PKH2-PCLgreen MΦs (top left quadrant). *P ≤ .05 and **, P ≤ .01, determined by Bonferroni t test with data expressed as mean plus or minus SEM.
rM mediates postresolution homeostasis. Mice were injected intravenously with clodronate-filled liposomes followed by zymosan (0.1 mg). Inflammation was first determined 4 hours later showing a significant increase over controls of (A) PMNs at onset followed by a confirmed reduction in (B) macrophages during resolution (48 hours) concomitant with a surprising reduction in (C) PMN numbers during this phase, suggesting that when phagocytosing macrophage are limited in numbers, PMNs may be efficiently cleared by parenchymal/stromal cells. However, (D) T- and B-lymphocyte numbers were also significantly reduced, revealing that rM cells are central to recruiting postresolution innate-type lymphocytes, which we have shown to be critical for postinflammation susceptibility to superinfection and restoration of tissue homeostasis.11 (E) We identified that an rM COX 2–derived prostanoid mediated these effects as NS-398 at 10 mg/kg dosed orally during resolution (48 and 60 hours) impaired CD3 cell repopulation at 72 hours. Moreover, (F) adoptively transferring PKH26-PCLred rM along with equal numbers of PKH2-PCLgreen M1 cells to a murine peritoneal cavity revealed that after 24 hours rM cells remained in the peritoneum, whereas M1 displayed a greater clearance rate, suggesting that rM cells possess a greater propensity to remain in the peritoneum to elicit their prohomeostatic functions, with the dotted line denoting the original numbers of MΦs injected. (G) FACS dot plot to prove the successful transfer of differentially labeled MΦs and illustrating the greater number of PKH26-PCLred MΦs (bottom right quadrant) compared with fewer PKH2-PCLgreen MΦs (top left quadrant). *P ≤ .05 and **, P ≤ .01, determined by Bonferroni t test with data expressed as mean plus or minus SEM.
Discussion
Resolution of acute inflammation is increasingly reported as an active event typified by the cessation of PMN trafficking, proinflammatory mediator catabolism, as well as apoptosis and phagocytosis collectively allowing stromal cells and parenchymal tissues of the injured site to resume their normal physiological function.7-10 Macrophages play an important role in this setting, helping to remove effete leukocytes and cellular debris, regulating tissue debridement, and eliciting wound healing.36,37 In innate immune responses to infection or injury, it is believed that macrophage phenotype is critical in determining whether the inflamed site resolves or progresses to chronic inflammation or undergoes collateral tissue injury. Despite this, the phenotype of proresolution macrophages is not known. To redress this, we have shown that compared with macrophages taken from a peritonitis that progressed to systemic inflammation and that exhibited a classical M1 phenotype, macrophages harvested from the resolving phase of acute peritonitis possess a unique phenotype. These resolution-phase macrophages are generally M2 in nature as they express mannose receptor and synthesize IL-10 and arginase 1 but also express markers typical of M1 macrophages (ie, COX 2 and iNOS). The latter interestingly produces comparatively modest amounts of NO (1-5 μM) despite the extracellular levels of arginine (approximately 20-30 μM) in the inflammatory milieu. Indeed, these respective phenotypes were robust in situ and long lived, as rMs from day 6 or 9 postresolution cavities continued to elaborate IL-10 and responded ex vivo with quantitatively more IL-10 and COX 2 but not TNFα when treated with defined stimuli (TLR2, TLR4, TLR6, or NOD 2 receptor agonists; the latter is not shown). Therefore, we report these unique cells as a hybrid of both definitions, referring to them as proresolution or rM macrophage. Certainly, although macrophage heterogeneity is well recognized to be diverse,2 the M1/M2 classification has arisen largely from the results of in vitro experiments and it is more likely that macrophage phenotypes occupying sites of inflammation may be less defined. Indeed, deviations from this nomenclature were reported in other studies where, for instance, murine macrophages infected with Streptococcus pyogenes exhibit the classical M1 response but without iNOS.38 Equally, F4/80+Gr1+ macrophages with T cell–suppressive activity and that are not M2 can be triggered in mice infected with schistosome egg glycans.39,40 Incidentally, rM cells in this current study that are F4/80 positive do not express the LY6C/G antigen (data not included). Therefore, it is this deviation from the in vitro–derived definitions that underlines the complexity of classifying macrophages into too tight a category, thereby complying with the literature2 that M2 cells have greater heterogeneity that M1 cells. In this regard, we suspect that in addition to the phenotype described here in the context of peritonitis, the phenotype of macrophages involved in the resolution of other immune responses needs to be evaluated and may be both tissue and stimulus specific.
The ability of macrophages to change phenotype during the course of inflammation is an emerging concept with an increasing body of literature suggesting that it might be a common theme in many tissues and disease states. For example, in experimental liver fibrosis, the same macrophages producing TNFα and TGFβ1 during the inflammatory peak and that drove the disease pathogenesis were involved in tissue regeneration during the repair phase.41 Similarly, macrophages from the adipose tissue of lean mice, which are largely M2 and expressing F4/80+CD11c+, are changed upon diet-induced obesity to express the M1 genes, TNFα and iNOS.42 Given the impact M2 macrophages have on inflammatory disease outcome—being central in ameliorating experimental allergic encephalitis43 and experimental chronic inflammatory renal disease44 and in conferring protection in the case of parasite-induced immune suppression of certain Th1-type diseases45 —understanding the flexible nature of macrophage phenotype would allow for greater pharmacological intervention to tailor inflammatory responses in an appropriate fashion. On this theme, we found that the potent immunomodulator, cAMP,20 is elevated in rMs, being comparatively lower in M1 cells. Moreover, increasing cAMP in M1 macrophages reduced TNFα secretion as well as their ability to kill S aureus while elevating COX 2 and iNOS, properties identical to rM cells. Conversely, inhibiting cAMP in rM cells changed their phenotype to that akin to M1 cells (ie, elaborating proinflammatory cytokines and increasing their bactericidal properties). Indeed, a well-documented consequence of catecholamines, used to treat hypotension associated with septic shock, includes stimulation of bacterial growth.46,47 As catecholamines elevate cAMP in cells of the innate immune system,48,49 it is tempting to speculate that increases in bacterial growth by catecholamines in the clinical literature may result, at least in part, from the formation of a bactericidal-naive rM phenotype. Thus, cAMP is at the center of controlling the phenotype of inflammatory macrophages in a manner that differentially regulates bacteria killing and cytokine release.
However, manipulating cAMP and therefore macrophage phenotype in vivo to resolve ongoing inflammation, as defined by PMN clearance concomitant with lymphocyte repopulation,11 is more ambiguous. For instance, transferring rM cells to mice bearing 10 mg zymosan failed to trigger resolution in these animals. It transpired that the phenotype of adoptively transferred rMs was altered by the inflammatory environment of ongoing inflammation to that resembling M1 cells, explaining the lack of efficacy of rM cells in these experiments. In contrast, the phenotype of M1 cells in inflammation elicited by 10 mg zymosan was partially altered toward rMs by cAMP-elevating agents, resulting in reduced TNFα, reflecting results obtained from in vitro reprogramming experiments. However, unlike the well-established anti-inflammatory effects of rolipram and A2A receptor agonists when given prophylactically at onset50,51 (D.W.G., unpublished data, March 2008), elevating cAMP during established inflammation did not reduce peritoneal leukocyte numbers. Thus, although pharmacological manipulation of cAMP brings about a change in macrophage phenotype during established inflammation with implications for the resolution of systemic inflammation, this is insufficient to trigger leukocyte clearance. These results suggest that soluble factors that drive ongoing inflammation predominate over proresolution factors. For this reason, we sought an alternative strategy to discern the role of rMs in self-limiting acute inflammation.
To this end, macrophages were depleted using clodronate-filled liposomes, revealing that despite a significant increase in PMNs at onset over controls, PMNs were significantly lower in clodronate-treated animals compared with rM-sufficient mice. This implies that in the presence of limiting rM numbers, PMNs can be efficiently cleared leading to resolution, with lymphatic drainage or phagocytosis by stromal cells representing likely alternative clearance routes for PMNs under these conditions. However, a consequence of limiting rM numbers was failed repopulation of resolution-phase lymphocytes, which we have shown recently to be a marker of postinflammation restoration of tissue physiology and essential for controlling innate immune–mediated responses to secondary infection.11 Therefore, we propose that cAMP is elevated consequent to resolution once signals that drive inflammation are dampened. And although it controls the balance of proinflammatory versus anti-inflammatory cytokines, cAMP is not required for leukocyte clearance. Once resolution processes are under way, COX 2–expressing rM cells, as a result of elevated cAMP, restore postinflammation homeostasis to equip injured tissue with the necessary cellular armamentarium (innate-type lymphocytes) to mount subsequent and appropriately tempered inflammatory events.
In conclusion, we have identified the phenotype of rMs as generally M2 but with characteristics of proinflammatory, M1 cells. rMs appear dispensable for PMN clearance during resolution but are essential for restoring tissue homeostasis to combat future infections.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
D.W.G. is a Wellcome Trust–funded Career Development Fellow, and J.B. is a recipient of a GlaxoSmithKline-funded project grant.
Wellcome Trust
Authorship
Contribution: D.W.G., J.B., and S.F. designed research, analyzed data, and wrote the paper, along with J.B. who also carried out the research; I.E., J.N., M.S., M.C., and I.T. carried out experiments and provided essential experimental material; and P.C.-N., S.F., and N.R. supplied essential experimental tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: D. W. Gilroy, Centre for Clinical Pharmacology and Therapeutics, Division of Medicine, 5 University Street, University College London, London WC1E 6JJ, United Kingdom; e-mail: d.gilroy@ucl.ac.uk.