Abstract
Anti-CD20 monoclonal antibodies (mAbs) are classified into type I (rituximab-like) or type II (tositumomab-like) based on their ability to redistribute CD20 molecules in the plasma membrane and activate various effector functions. To compare type I and II mAbs directly in vivo and maximize Fc effector function, we selected and engineered mAbs with the same mouse IgG2a isotype and assessed their B-cell depleting activity in human CD20 transgenic mice. Despite being the same isotype, having similar affinity, opsonizing activity for phagocytosis, and in vivo half-life, the type II mAb tositumomab (B1) provided substantially longer depletion of B cells from the peripheral blood compared with the type I mAb rituximab (Rit m2a), and 1F5. This difference was also evident within the secondary lymphoid organs, in particular, the spleen. Failure to engage complement did not explain the efficacy of the type II reagents because type I mAbs mutated in the Fc domain (K322A) to prevent C1q binding still did not display equivalent efficacy. These results give support for the use of type II CD20 mAbs in human B-cell diseases.
Introduction
Rituximab is now part of the standard treatment for many B-cell malignancies and is finding utility in a range of autoimmune diseases where depletion of normal B cells, although not as yet explained mechanistically, appears highly beneficial.1,2 Despite such success, rituximab remains a prototype chimeric human/mouse monoclonal antibody (mAb), which is not ideally designed for clinical use and does not benefit from the latest technologies available for engineering more potent reagents.3 Not surprisingly, the pursuit of improved reagents to replace rituximab is intense, with several candidates currently under clinical evaluation4 (Table 1). Most have been selected and engineered to provide a range of potential benefits, including increased affinity, reduced immunogenicity, and improved antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). As yet it is not clear which, if any, of these improvements will deliver improved efficacy in the clinic. However, most evidence supports the notion that FcR-based mechanisms are the most critical for therapeutic success,5 in particular, data showing that patients with high, rather than low, affinity variants of FcγRIIIA (CD16) benefit most from rituximab treatment.5 Consequently, it is the Fc:FcR interaction that is most often targeted for improvement in new mAbs.6-8
Selected anti-CD20 mAbs in clinical development
| Antibody . | Antibody type . | Format . | Sponsor . | Stage of development . |
|---|---|---|---|---|
| Ocrelizumab (PRO70769)45 | I | Humanized IgG1 (2H7) with increased binding to FcγRIIIa and decreased CDC | Genentech/Roche/Biogen | Phase 3 trials for NHL and autoimmune conditions |
| Ofatuzumab (HuMax CD20) | I | Human (Ig transgenic mice) IgG1 with high CDC | Genmab AC/GSK | Phase 3 trials for NHL and autoimmune conditions |
| TRU-015 | I | SMIP with high affinity for CD20, strong ADCC activity, and weak CDC | Trubion Pharrma/Wyeth | Phase 1/2 in in RA |
| Veltuzumab (Ah20) | I | Humanized (similar binding and activity to rituximab) | Immunomedics | Phase 1/2 in NHL and ITP (including delivery by subcutaneous route) |
| AME-133v46 | I | Humanized IgG1 selected for increased binding to CD20 and FcRIIIa leading to augmented ADCC | Applied Molecular Evolution/Eli Lilly | Phase 1/2 in relapsed NHL |
| PRO131921 (version 114) | I | Humanized IgG1 version of LyB-1 with elbow mutations and modified Fc glycosylation giving augmented ADCC and PCD | Genentech | Phase 1/2 in CLL and NHL |
| GA101 | II | Third-generation anti-CD20 engineered from LyB-1 with optimized Fc activity (ADCC) | Glycart/Roche | Phase 1 initiated |
| Antibody . | Antibody type . | Format . | Sponsor . | Stage of development . |
|---|---|---|---|---|
| Ocrelizumab (PRO70769)45 | I | Humanized IgG1 (2H7) with increased binding to FcγRIIIa and decreased CDC | Genentech/Roche/Biogen | Phase 3 trials for NHL and autoimmune conditions |
| Ofatuzumab (HuMax CD20) | I | Human (Ig transgenic mice) IgG1 with high CDC | Genmab AC/GSK | Phase 3 trials for NHL and autoimmune conditions |
| TRU-015 | I | SMIP with high affinity for CD20, strong ADCC activity, and weak CDC | Trubion Pharrma/Wyeth | Phase 1/2 in in RA |
| Veltuzumab (Ah20) | I | Humanized (similar binding and activity to rituximab) | Immunomedics | Phase 1/2 in NHL and ITP (including delivery by subcutaneous route) |
| AME-133v46 | I | Humanized IgG1 selected for increased binding to CD20 and FcRIIIa leading to augmented ADCC | Applied Molecular Evolution/Eli Lilly | Phase 1/2 in relapsed NHL |
| PRO131921 (version 114) | I | Humanized IgG1 version of LyB-1 with elbow mutations and modified Fc glycosylation giving augmented ADCC and PCD | Genentech | Phase 1/2 in CLL and NHL |
| GA101 | II | Third-generation anti-CD20 engineered from LyB-1 with optimized Fc activity (ADCC) | Glycart/Roche | Phase 1 initiated |
Data modified from Maloney.44
SMIP indicates small modular immunopharmaceutical drug composed of human IgG1 Fc and hinge regions (hinge, CH2, and CH3) linked directly to an anti-CD20 scFv.
The role of complement in rituximab therapy remains controversial. Although complement is consumed during rituximab treatment9 and on some occasions cells remaining or emerging after treatment appear to have been selected to express increased levels of complement defense molecules,10,11 it has also been shown that the expression of complement defense molecules on tumor cells does not predict clinical outcome, and no correlation exists between in vitro complement sensitivity and subsequent therapeutic response.12 Moreover, recent findings indicate that complement activation may indeed be detrimental to the therapeutic activity of rituximab. First, the C3b deposition after rituximab engagement of CLL cells is thought to promote loss of CD20 and thereby therapeutic efficacy through the shaving reaction.13 Second, Wang et al have now suggested that deposited C3b may inhibit the interaction between the Fc region of rituximab and CD16 on NK cells, thereby limiting its ability to induce NK activation.14 Thus, complement (inactivated C3b), rather than promoting tumor destruction, could actually impair the therapeutic efficacy of rituximab by promoting loss of CD20 and blocking ADCC.
We previously defined anti-CD20 mAbs as either type I or II, based on their ability to redistribute CD20 into lipid rafts and their potency in various effector assays, such as CDC, homotypic adhesion, and programmed cell death (PCD).15-17 These experiments also indicated that the rarer type II mAbs (such as tositumomab/B1), with their greater tendency to promote PCD but not CDC, are more effective at depleting malignant B cells in xenograft models.16 However, the inherent variability of xenograft growth and response to mAb make these models less than ideal to study FcR effector functions of rituximab, particularly as rituximab has human IgG1 constant regions, which will operate poorly with mouse effectors.18
To address these concerns and compare directly the in vivo efficacy of type I and II mAbs, we selected and engineered a range of mAbs with identical murine IgG2a constant regions (the most therapeutically effective isotype in the mouse) and assessed their B-cell-depleting ability in transgenic mice expressing human CD20 (hCD20) on their mature B cells.19 These experiments revealed that a type II mAb displayed significantly greater ability to deplete B cells than type I mAbs. In 2 strains of mice (BALB/c and C57Bl/6), a type II mAb was more potent both in the duration of B-cell depletion from the circulation and the extent of depletion in the secondary lymphoid organs. This enhanced activity was not related to differences in affinity, opsonization for phagocytosis, isotype, or half-life. Furthermore, using mutant IgG2a type I mAb, which lacked complement activating activity, we demonstrate that the ability of type I mAb to deplete B cells is not dependent on complement activation and importantly is not markedly improved when complement is not engaged.
Methods
Cells and animals
SU-DHL-4 and Raji cells were obtained from the European Collection of Cell Cultures and maintained in antibiotic-free RPMI (Invitrogen, Paisley, United Kingdom) containing 10% fetal calf serum, at 37°C, in 5% CO2. BALB/c and C57Bl/6 mice were originally obtained from Charles River (Margate, United Kingdom) and bred and maintained in local facilities. hCD20 transgenic mice have been described previously19 and were backcrossed onto BALB/c and C57Bl/6 backgrounds for 10 generations. C1q-deficient mice were obtained from Dr Aras Kadioglu (Leicester, United Kingdom) with permission from Professor Marina Botto (London, United Kingdom) and have been described before.20 Animal experiments were cleared through the local ethical committee at the University of Southampton and were performed under Home Office licenses PPL30/1269 and PPL30/2451.
hCD20 Tg B cells used for in vitro and in vivo targeting assays were obtained through negative selection using a B-cell isolation kit (MACS, Miltenyi Biotec, Auburn, CA) from the spleens of BALB/c mice.
Antibodies
Tositumomab (B1), 1F5, and WR17 (mouse antihuman CD37) mAbs are all of the mIgG2a isotype and have been described previously.15 Tositumomab and rituximab were kind gifts from Prof Tim Illidge (Manchester, United Kingdom) and Dr Luke Nolan (Southampton, United Kingdom). Rituximab m2a (Rit m2a) and the complement inactive K322A variant (Rit m2a K322A) were generated from patent published sequences using 1F5 heavy and light chain DNA as a template with the QuickChange Multi Site-Directed Mutagenesis kit (Stratagene, Cambridge, United Kingdom) according to the manufacturer's protocols, as was the K322A variant of 1F5. Antibody was produced using the 293F Freestyle system (Invitrogen) and purified on Protein A. The purity of mAb was assessed by electrophoresis (Beckman EP system, Beckman Coulter, Fullerton, CA) and lack of aggregation verified by HPLC.
Flow cytometry
Fluorescently conjugated Ab were purchased from BD Biosciences (San Jose, CA) or were conjugated in-house. Flow cytometry for surface binding was as described previously21 with samples assessed on a FACSCalibur and data analyzed with CellQuest Pro (BD Biosciences).
Effector functions
CDC was assessed using a rapid and simple propidium iodide exclusion flow cytometric assay detailed previously17 with either normal human serum prepared from normal volunteer blood or fresh rat serum used as the complement source. Briefly, targets (SU-DHL-4 or hCD20 Tg B cells) were incubated with mAb for 15 minutes at room temperature and then 20% serum added for 30 minutes at 37°C. Assessment of raft-redistribution was performed using a flow cytometric Triton X-100 insolubility assay as detailed previously.17 Homotypic adhesion was assessed by adding mAb (5-10 μg/mL) to cells in flat-bottomed plastic plates (Nalge Nunc International, Rochester, NY) and then examining them 4 to 24 hours later. Images were captured using an Olympus CKX41 microscope linked to a CC12 cooled camera. Opsonization potential for phagocytosis was assessed in vitro using hCD20 Tg B cells and thioglycollate-elicited macrophages as follows. First, 1 mL of 4% thiogycollate medium was injected intraperitoneally into BALB/c mice and after 5 days peritoneal macrophages were harvested and pooled. Macrophages were resuspended in X-VIVO 15 medium (Lonza Nottingham, Nottingham, United Kingdom) at 2.5 × 105 CD11b-positive macrophages/mL, plated into a 48-well tissue culture plate (Nalge Nunc International), and incubated for 2 hours at 37°C. Nonadherent cells were then removed and replaced with fresh medium. Macrophages were then rested for a further 30 minutes before use in the phagocytosis assay. For targets, hCD20 Tg B cells were labeled with 5 μM of carboxyfluorescein succinimidyl ester (CFSE) for 10 minutes at 37°C, then washed and resuspended at 1 × 107 cells/mL. CD20 mAb (100 μg/mL) was then added to the cells for 30 minutes at room temperature before addition to the macrophages. Cells were then incubated at 37°C and after 1 hours labeled with 10 μg/mL of phycoerythrin-anti-CD11b antibody for 15 minutes at room temperature to distinguish the macrophages, washed, and then analyzed by flow cytometry. The percentage phagocytosis was determined by calculating the proportion of cells that stained double positive for CFSE and CD11b.
B-cell depletion experiments
To determine B-cell depletion in hCD20 Tg mice, animals were treated with a single intravenous dose of mAb (250 μg) and the number of B cells remaining in the blood or other organs assessed by flow cytometry or immunohistochemistry. Residual B cells in treated mice were plotted as percentage of B cells in control mice treated with an irrelevant isotype-matched mAb (WR17) or phosphate-buffered saline alone.
To determine the depletion of hCD20 Tg B cells adoptively transferred into recipient animals, whole splenocyte suspensions from hCD20Tg mice (BALB/c background) and wild-type BALB/c mice were labeled with 2 μM and 0.4 μM CFSE, respectively, mixed to give a ratio of approximately 1:1, and injected intravenously into recipient mice (1-3 × 106/mouse). Mice received 100 μg of mAb intravenously 24 hours later, and spleens were harvested 16 hours later. Spleen suspensions were then labeled with APC-anti-CD19 mAb to identify B cells and samples analyzed by flow cytometry to determine the ratio of target to nontarget CFSE-labeled cells.
Immunohistochemistry
For immunohistochemistry, 10-μm frozen sections of tissues were fixed in acetone, blocked with 5% normal goat serum, and labeled with rat anti-CD45R/B220 (clone RA3-6B2, BD Biosciences) and hamster anti-CD3 (clone 1452C11; in house) followed by biotinylated goat antirat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa Fluor 488-conjugated goat antihamster IgG, and streptavidin Alexa Fluor 635 (Invitrogen). Sections were counterstained with Sytox-orange (Invitrogen) and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Negative controls included omission of primary Ab and replacement with an irrelevant, isotype-matched antibody. Images were collected sequentially on a Leica SP2 CLSM using 488 nm, 543 nm, and 633nm laser lines and a pinhole equivalent to 1 Airy disc.
Statistical analysis
To compare differences between the experimental groups, a 2-tailed t test was performed using GraphPadPrism software.
Results
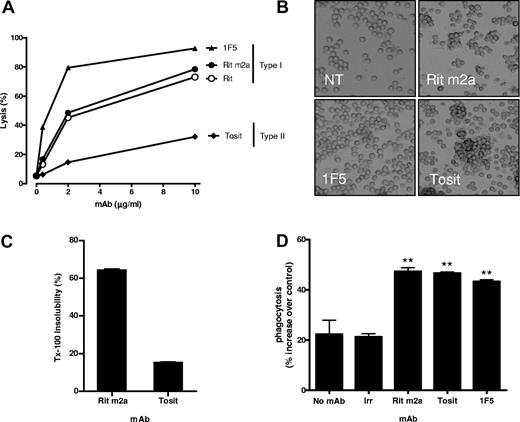
To directly compare type I and II mAbs in vivo and maximize engagement of FcR-bearing effector systems,22 we first engineered rituximab to carry the same mouse IgG2a isotype as tositumomab (B1) and 1F5 and compared the parental chimeric and mouse variant in CDC. Converting rituximab to mouse IgG2a (Rit m2a) did not influence its binding (not shown) or its type I status, and it allowed potent CDC activity at doses as low as 2 μg/mL (Figure 1A). In contrast, the type II mAb, tositumomab, despite being the same isotype and binding to the same region of CD20,23,24 had minimal CDC activity even at saturating concentrations in keeping with previous work.17
Characterization of anti-CD20 mAb. (A) SU-DHL-4 cells at 1 × 106/mL were incubated with mAb at 0.4, 2, and 10 μg/mL at room temperature for 15 minutes. Normal human serum was added as a source of complement, cells incubated at 37°C for 30 minutes, then propidium iodide added, and cells analyzed for cell lysis by flow cytometry. Data are representative of 3 independent experiments. (B) Examples of homotypic adhesion produced by the different mAbs after incubation with Raji cells for 4 hours at 37°C. Cells in tissue culture plates were viewed with an Olympus CKX21 inverted microscope (Olympus, Watford, United Kingdom) using a 10×/0.25 PH lens. Images were acquired using a CCL2 digital cooled camera (Olympus) and were processed with Cell B (Olympus Soft imaging solutions) and Adobe Photoshop version CS2 software (Adobe Systems, San Jose, CA). Original magnification 20×. (C) Rit m2a or tositumomab (Tosit) was added to hCD20 Tg B cells for 30 minutes on ice, and then the proportion of mAb that had redistributed to the Triton X-100-insoluble fraction was determined by flow cytometry. Bars show the mean plus or minus SEM for triplicate samples. (D) hCD20 Tg splenocytes were labeled with CFSE, incubated with nothing (no mAb), and irrelevant mAb (WR17), Rit m2a, 1F5, or tositumomab (Tosit) and subjected to a 1-hour phagocytosis assay with thioglycollate-activated peritoneal macrophages. Bars represent the mean plus or minus SEM for triplicate samples.
Characterization of anti-CD20 mAb. (A) SU-DHL-4 cells at 1 × 106/mL were incubated with mAb at 0.4, 2, and 10 μg/mL at room temperature for 15 minutes. Normal human serum was added as a source of complement, cells incubated at 37°C for 30 minutes, then propidium iodide added, and cells analyzed for cell lysis by flow cytometry. Data are representative of 3 independent experiments. (B) Examples of homotypic adhesion produced by the different mAbs after incubation with Raji cells for 4 hours at 37°C. Cells in tissue culture plates were viewed with an Olympus CKX21 inverted microscope (Olympus, Watford, United Kingdom) using a 10×/0.25 PH lens. Images were acquired using a CCL2 digital cooled camera (Olympus) and were processed with Cell B (Olympus Soft imaging solutions) and Adobe Photoshop version CS2 software (Adobe Systems, San Jose, CA). Original magnification 20×. (C) Rit m2a or tositumomab (Tosit) was added to hCD20 Tg B cells for 30 minutes on ice, and then the proportion of mAb that had redistributed to the Triton X-100-insoluble fraction was determined by flow cytometry. Bars show the mean plus or minus SEM for triplicate samples. (D) hCD20 Tg splenocytes were labeled with CFSE, incubated with nothing (no mAb), and irrelevant mAb (WR17), Rit m2a, 1F5, or tositumomab (Tosit) and subjected to a 1-hour phagocytosis assay with thioglycollate-activated peritoneal macrophages. Bars represent the mean plus or minus SEM for triplicate samples.
We have reported previously that type I mAbs, such as rituximab and 1F5, show little tendency to trigger homotypic adhesion compared with type II mAbs15 but have the ability to redistribute CD20 into lipid rafts whereas type II mAbs do not.17 The type I status of Rit m2a was confirmed by its inability to promote strong homotypic adhesion (Figure 1B) and its ability to redistribute CD20 into membrane rafts (Figure 1C) compared with tositumomab (B1). In addition, Rit m2a also displayed approximately twice the level of surface binding on hCD20 B cells compared with tositumomab (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), in keeping with its type I nature and demonstrating that hCD20 behaves as expected on the surface of mouse B cells.
Next, we assessed the ability of the various mAbs to evoke phagocytosis in vitro using hCD20 Tg splenocytes as targets and thioglycollate-elicited peritoneal macrophages as the effector cells. These results demonstrate that Rit m2a, 1F5, and tositumomab all induced equivalent levels of phagocytosis, as anticipated given their strong binding and murine IgG2a isotypes (Figure 1D).
Having characterized the mAbs in vitro, we next compared their ability to deplete normal circulating B cells in hCD20 transgenic C57Bl/6 (Figure 2A) and BALB/c (Figure 2B) mice. In these mice, hCD20 was only detected on the surface of CD19+ B lymphocytes (Figure S2). Although similar, the expression of hCD20 was routinely higher on BALB/c B cells (Figure S2).
In vivo circulating B-cell depletion with antihuman CD20 mAbs. Transgenic hCD20 mice on C57Bl/6 (A) or BALB/c (B) backgrounds received 250 μg of anti-hCD20 mAb or an isotype-matched control (WR17) intravenously on day 0. The numbers of circulating B cells were then assessed by CD19 and B220 staining of peripheral blood and flow cytometry. The results are expressed as percentage of B cells observed at time 0, n ≥ 4 from at least 2 to 4 independent experiments.
In vivo circulating B-cell depletion with antihuman CD20 mAbs. Transgenic hCD20 mice on C57Bl/6 (A) or BALB/c (B) backgrounds received 250 μg of anti-hCD20 mAb or an isotype-matched control (WR17) intravenously on day 0. The numbers of circulating B cells were then assessed by CD19 and B220 staining of peripheral blood and flow cytometry. The results are expressed as percentage of B cells observed at time 0, n ≥ 4 from at least 2 to 4 independent experiments.
In both strains, a single dose of the type II mAb (250 μg) produced significantly longer depletion of circulating B cells than did the type I mAb (tositumomab compared with Rit m2a and 1F5; P < .001 comparing the day at which 50% of the B cells had returned; Figure S3; and data not shown). This enhanced depletion did not correlate with any difference in binding affinity (Rit m2a (5-8 × 10−9 M), tositumomab (3-6 × 10−9 M), 1F5 (1-3 × 10−8 M)17 (and data not shown). Similarly, biologic half-life did not explain the differences because both types of mAb persist equally well in the hCD20 Tg mice (data not shown). In the case of BALB/c (Figure 2B), the duration of B-cell depletion achieved with the type II mAb was approximately 60 days, more than twice that seen with the type I reagents. It is also noteworthy that depletion of circulating B cells with type I mAb was more effective in BALB/c compared with C57Bl/6 mice (Figure 2A,B) potentially because of the lower overall proportion of B cells in both the periphery and secondary lymphoid organs (Figure S4).
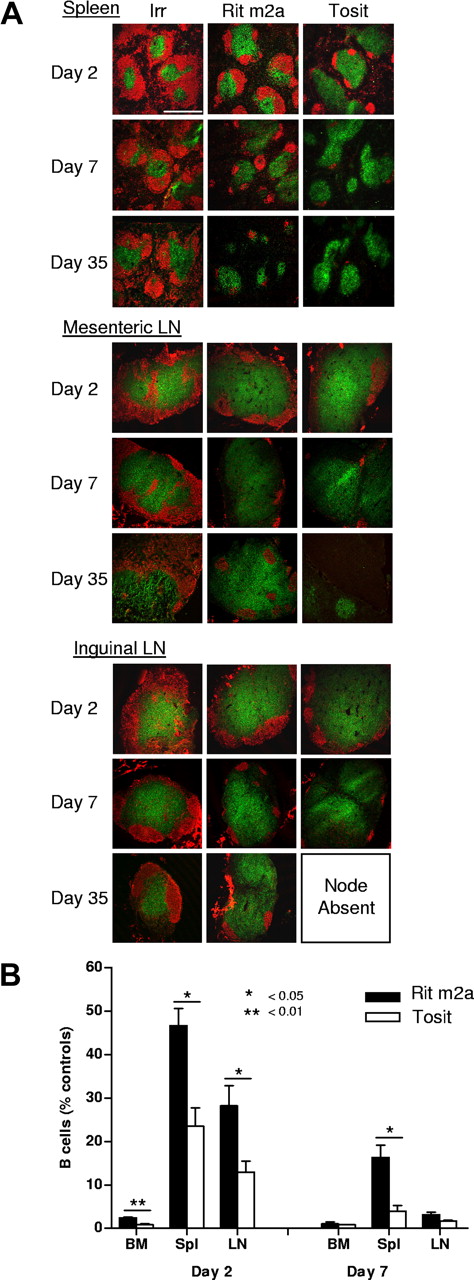
Having established the greater efficacy of the type II mAb tositumomab in depleting B cells from the blood, next we assessed B-cell depletion from the secondary lymphoid organs. Here, we found similar results, with superior clearance of B cells from bone marrow, lymph node, and spleen with tositumomab compared with Rit m2a (Figure 3) and 1F5 (not shown) out to day 35. The marked difference in performance was particularly evident in the spleen where the type II mAb was able to rapidly and extensively deplete B cells (Figure 3B), resulting in approximately 5% B220+ cells by day 7 after a single dose of tositumomab, compared with almost 20% with Rit m2a (P < .05). Depletion of B cells within the LN and bone marrow was also more marked with type II mAb, although these differences were more marked on day 2 than on day 7.
Depletion of B-cell subsets in the lymphoid compartment. (A) Immunohistochemistry of B-cell depletion in the spleen and mesenteric and inguinal lymph nodes. Transgenic hCD20 mice (BALB/c) received 250 μg of antihuman CD20 mAb or an isotype-matched control mAb (WR17) intravenously and killed on days 2 or 7. Tissues were harvested, embedded, and frozen, and sections stained for B cells (B220; red) and T cells (CD3; green). Scale bar represents 500 μm. Slides were viewed with a Leica SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany) using a 10× dry plan fluotar lens with a 1.5× optical zoom with Vectashield mount (Vector Labs, Burlingame, CA). Images were acquired using Leica Confocal Software v2.61 and were processed with Adobe Photoshop version CS2 software (Adobe Systems). (B) Mice were treated for 2 or 7 days as indicated in panel A, then cells isolated from the bone marrow (BM), spleen (Spl), or lymph node (LN). The proportion of B cells remaining in these organs was determined by flow cytometry, compared with controls and expressed as a percentage. Bars represent mean plus or minus SEM (n = 3 from 2 independent experiments).
Depletion of B-cell subsets in the lymphoid compartment. (A) Immunohistochemistry of B-cell depletion in the spleen and mesenteric and inguinal lymph nodes. Transgenic hCD20 mice (BALB/c) received 250 μg of antihuman CD20 mAb or an isotype-matched control mAb (WR17) intravenously and killed on days 2 or 7. Tissues were harvested, embedded, and frozen, and sections stained for B cells (B220; red) and T cells (CD3; green). Scale bar represents 500 μm. Slides were viewed with a Leica SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany) using a 10× dry plan fluotar lens with a 1.5× optical zoom with Vectashield mount (Vector Labs, Burlingame, CA). Images were acquired using Leica Confocal Software v2.61 and were processed with Adobe Photoshop version CS2 software (Adobe Systems). (B) Mice were treated for 2 or 7 days as indicated in panel A, then cells isolated from the bone marrow (BM), spleen (Spl), or lymph node (LN). The proportion of B cells remaining in these organs was determined by flow cytometry, compared with controls and expressed as a percentage. Bars represent mean plus or minus SEM (n = 3 from 2 independent experiments).
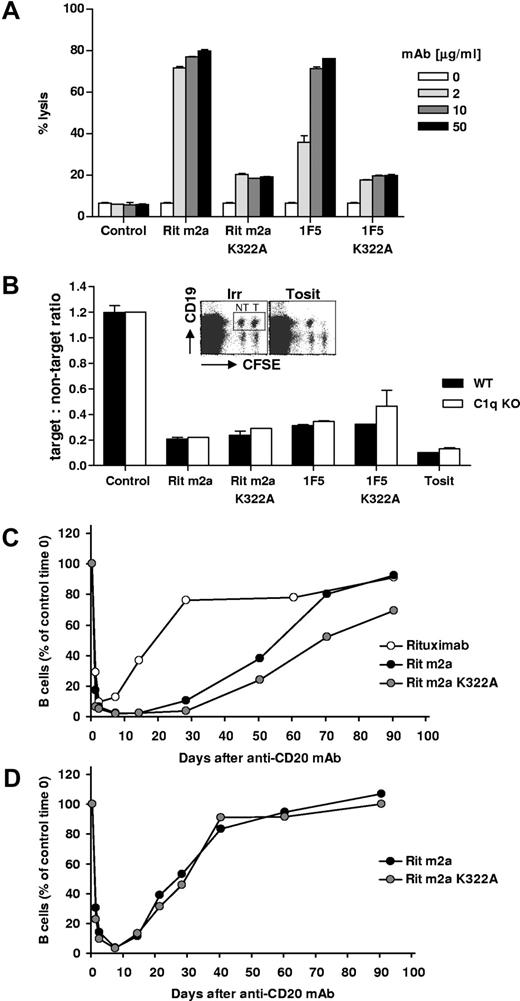
The role of complement as an effector mechanism in mAb therapy remains controversial with evidence that it may make both positive and negative contributions to rituximab therapy (reviewed by Glennie et al4 ). If complement were detrimental to Ab immunotherapy, then it could explain the reduced efficacy seen with complement-activating type I mAb in the current study. Therefore, next we assessed the role of complement on type I mAb using mutant (K322A) versions of Rit m2a and 1F5, which lack the ability to bind C1q and consequently to activate the classical complement pathway. After confirming that the K322A versions of these mAbs were greatly impaired in their ability to activate lytic complement against the hCD20 Tg (Figure 4A) or SU-DH-L4 cells (data not shown), we assessed their activity in B-cell depletion. Initially, this was achieved using a sensitive adoptive transfer assay in which CSFE-labeled splenic B cells (equal numbers of WT and hCD20 Tg) were transferred to either wild-type or complement-deficient (C1q−/−) mice before treatment with intact or K322A mutated mAbs. Under these conditions, the survival of the transferred hCD20 Tg B cells could be assessed relative to the nontarget, WT, B cells in individual mice. Figure 4B shows that, regardless of whether complement was systemically absent (C1q−/−) or specifically lacking in the mAb (K322A mutation), the type I mAb could not match the type II mAb, tositumomab, in depleting B cells. Furthermore, in this in vivo assay, the ability to activate complement did not appear to help nor hinder the activity of either type I mAb as both 1F5 and Rit m2a retained the same level of activity with and without the ability to engage complement. Thus, preventing a type I mAb from activating complement does not increase its potency to that of a type II mAb.
Role of complement in B-cell depletion. (A) Various concentrations (2-50 μg/mL) of Rit m2a and 1F5 or their K322A mutant forms were bound to hCD20 Tg B cells before the addition of normal rat serum as a source of complement for 30 minutes at 37°C. Cell lysis was then determined by flow cytometry. Bars represent the mean and range for duplicate samples and are representative of 3 independent experiments. (B) hCD20 Tg (T) or non-Tg (NT) BALB/c mouse splenocytes were labeled with high or low levels of CFSE, respectively, pooled and injected into WT or C1q−/− recipient mice; 24 hours later, mice were injected with various mAbs (100 μg, intravenously), and 16 hours after that splenocytes were labeled with anti-CD19 mAb and were assessed by flow cytometry for the relative proportion of surviving hCD20 Tg B cells expressed as the target:nontarget ratio. Bars represent mean and range for duplicate samples. Transgenic hCD20 BALB/c (C) or C57Bl/6 (D) mice received 250 μg of antihuman CD20 mAb (rituximab, Rit m2a, 1F5) or mAb lacking complement activity (K322A mutation) intravenously on day 0. The numbers of circulating B cells was then assessed from peripheral blood by CD19 and B220 staining and flow cytometry. The results are expressed as percentage of B cells observed at time 0, n ≥ 4.
Role of complement in B-cell depletion. (A) Various concentrations (2-50 μg/mL) of Rit m2a and 1F5 or their K322A mutant forms were bound to hCD20 Tg B cells before the addition of normal rat serum as a source of complement for 30 minutes at 37°C. Cell lysis was then determined by flow cytometry. Bars represent the mean and range for duplicate samples and are representative of 3 independent experiments. (B) hCD20 Tg (T) or non-Tg (NT) BALB/c mouse splenocytes were labeled with high or low levels of CFSE, respectively, pooled and injected into WT or C1q−/− recipient mice; 24 hours later, mice were injected with various mAbs (100 μg, intravenously), and 16 hours after that splenocytes were labeled with anti-CD19 mAb and were assessed by flow cytometry for the relative proportion of surviving hCD20 Tg B cells expressed as the target:nontarget ratio. Bars represent mean and range for duplicate samples. Transgenic hCD20 BALB/c (C) or C57Bl/6 (D) mice received 250 μg of antihuman CD20 mAb (rituximab, Rit m2a, 1F5) or mAb lacking complement activity (K322A mutation) intravenously on day 0. The numbers of circulating B cells was then assessed from peripheral blood by CD19 and B220 staining and flow cytometry. The results are expressed as percentage of B cells observed at time 0, n ≥ 4.
Finally, we confirmed in the hCD20 Tg mice that the B-cell-depleting activity of Rit m2a K322A was similar to that of the unmutated Rit m2a version (Figure 4C,D), with perhaps a suggestion that the complement blocked variant (Rit m2a K322A) might be slightly more active in sustaining the removal of B cells, particularly in BALB/c mice. However, in none of the experiments did the activity of Rit m2a K322A come close to that of tositumomab. Similar results were observed with the 1F5 K322A variant mAb, with an activity level that was close to, or perhaps slightly better than, the original 1F5, but again falling far short of the activity seen with the type II mAb (data not shown; Figure S3). Therefore, in this model, complement activation is not sufficient to explain the difference between type I and II mAbs. As expected, compared with the Rit m2a, the huIgG1, rituximab, was considerably less effective in depleting circulating cells, presumably because of its inferior recruitment of natural effectors (Figure 4C).18
Discussion
Here we have shown that a type II anti-CD20 mAb, tositumomab, is far more potent than type I, rituximab-like, mAbs at depleting B cells in 2 strains of hCD20 transgenic mice. Importantly, using fully syngeneic systems and mAbs of the same mouse IgG2a isotype, we have been able to circumvent problems that typically confound in vivo comparisons of mAb efficacy, such as isotype differences and the problems associated with xenograft models in immune-deprived mice. The mAbs used in this study were shown to be of type I or II using CDC, homotypic adhesion, and raft redistribution assays, and in the case of rituximab to retain its type I character despite being converted from human IgG1 to mouse IgG2a. Interestingly, the interaction of CD20 mAb with macrophage Fc receptors did not appear to be influenced by redistribution into lipid rafts and all the CD20 mAb promoted similar levels of phagocytosis of opsonized hCD20 Tg B cells by peritoneal macrophages.
The superior B-cell-depleting activity of the type II mAb in vivo was demonstrated in 2 mouse strains, although it is noteworthy that circulating B cells in C57Bl/6 mice were more resistant to depletion by type I mAbs than those in BALB/c mice (Figure 2). Differences in the ease of B-cell depletion between mouse strains has been noted previously. For example, Ahuja et al19 found that B cells in autoimmune-prone MRL/MpJ-Fas mice were particularly resistant to depletion by rituximab, requiring large, regular doses to induce substantial B-cell depletion. Interestingly, B cells appear more refractory to depletion in several autoimmune-prone mice strains.19,25 It is not clear at this stage whether such results are a product of the autoimmune disease itself or the mouse strain in which such diseases arise, for example, as the byproduct of certain FcR polymorphisms, such as reported for mouse FcγRIIb.26
The difference in efficiency with which B cells were depleted in BALB/c and C57Bl/6 mice was most prominent when using the type I reagents. For example, a single treatment with Rit m2a cleared circulating B cells from BALB/c for close to 30 days, whereas in C57Bl/6 mice this depletion lasted for less than 14 days. Why B cells should be more resistant to depletion by type I reagents in C57Bl/6 mice is not clear. However, interestingly, C57Bl/6 mice have a greater proportion of circulating and tissue resident B cells (approximately double) and slightly lower expression of hCD20 than do the Tg BALB/c mice (Figures S2,S4), so the strain difference may simply reflect these factors. In contrast, tositumomab, being far more efficient at B-cell depletion, may be less influenced by B-cell numbers or CD20 density, resulting in its equivalent potency in both strains.
The role of complement as an effector in mAb therapy remains controversial, with evidence from a number of model systems indicating that it contributes to the efficacy of therapeutic mAbs, but with little direct evidence that it plays an important role in patients, particularly as a cytotoxic entity.4 Moreover, Wang et al recently provided data to indicate that complement activation, rather than contributing to treatment, might actually be detrimental to rituximab efficiency.27 Their work suggests that inactivated C3b deposition on rituximab-coated target cells can inhibit the interaction between the Fc region and CD16 on NK cells, thereby limiting the ability of rituximab to induce NK activation.27 If correct, this suggests that complement (inactivated C3b) could actually reduce FcR binding and effectively decrease ADCC. Presumably, any negative impact of complement on Fc:FcγR binding would apply to all types of FcγR, not just to CD16 as reported. In the current work, we examined the potential negative influence of complement using complement-inactive versions of rituximab (Rit m2a) and 1F5. We found that the therapeutic activity of the mutated Rit m2a K322A and 1F5 K322A was close to that of the parental mAb, with perhaps the suggestion of an improved activity in BALB/c mice, when complement was not available for activation. These results are important because they show 1) that complement plays little, if any, role as a CD20 mAb effector mechanism against normal B cells, and 2) that any negative impact of complement on ADCC is small and insufficient to explain the greater potency of the type II mAb.
Previously, we showed that complement was important for the therapeutic activity of type I mAbs in several xenograft model systems,16 an observation supported by others in both similar28 and different models,29-31 but difficult to reconcile with the current findings. Perhaps the simplest and most straightforward explanation comes from the differences in the cells targeted in the various studies. All of the studies where a clear positive role for complement has been reported used type I anti-CD20 mAb against small numbers of malignant B cells. In contrast, the majority of studies showing no prominent role for complement have studied the deletion of normal B cells using either a unique panel of antimouse CD20 mAb in wild-type mice,32,33 or, as here, antihuman CD20 mAb in hCD20 Tg mice.19,34 In addition to the obvious difference in the number of target cells in these 2 instances (the entire B-cell population vs low numbers of malignant human B cells), a principle difference may relate to their size and expression level of CD20. Malignant B cells are routinely blasted and larger than their normal counterparts, perhaps resulting in a higher surface expression of CD20. Because CDC (unlike ADCC) is critically dependent on the expression level of the target molecule,35 this may explain why hCD20 Tg B cells are more resistant to the effects of lytic complement than malignant human B cells. Alternatively, the differences between normal and malignant B cells might result from their sensitivity to Ab effector systems such as complement. For example, malignant human B cell lines are often routinely passaged in tissue-culture with heat-inactivated serum, which may lead to a down-regulation in complement defense molecules, thereby potentially explaining their increased sensitivity to lytic complement. An additional possible explanation is that the larger size of malignant B cells may reduce their ease of engulfment by macrophages. As such, tumor cells may rely more heavily on complement components, such as inactivated C3b, to opsonize them and promote phagocytosis.36-38 We should also not overlook the possibility that different Ab effector systems are used in different body compartments, which may allow complement to play a more prominent role in certain sites where cellular effectors are less available or perhaps where they become exhausted by high exposure to high numbers of Ab-coated targets (reviewed by Glennie et al4 ). Normal B cells, because of their tendency to circulate, may be removed by cellular effectors in a range of different compartments and consequently be less likely to exhaust any particular effector system. We are currently performing experiments to address these various possibilities.
Perhaps the most important finding of the current work is the higher potency of the type II mAb, tositumomab, relative to the type I mAbs. Tositumomab is currently only approved for human use as an Iodine-131 radio-immunoconjugate (Bexxar) in the treatment of relapsed low-grade or follicular non-Hodgkin lymphoma where it is extremely successful39,40 potentially through its ability to bypass apoptotic resistance.41 Unfortunately, to date, tositumomab has not been humanized and tested in patients as a naked mAb to allow direct comparison with rituximab. The explanation for its potency in the current investigation is not clear. We know that, other than leaving CD20 outside lipid rafts and having half as many epitopes available on B cells as type I reagents, tositumomab binds with typical affinity for a murine mAb.15,17 However, type II mAb are functionally distinct particularly in their ability to mediate homotypic adhesion and to promote PCD in a range of B cell lines. It is logical to think that this distinct functional activity is directly linked to the way they engage CD20, or more precisely its higher-order oligomers (eg, dimers/tetramers) in the B-cell membrane. The nature of such binding is intriguing because, although it remains bivalent, it must also explain the half-maximal binding of type II mAb and their failure to move CD20 into lipid rafts.15,17 Thus, although we struggle for a satisfactory explanation for these binding characteristics and speculate that epitope orientation may be critical, understanding this phenomenon could hold the key to explaining the superior efficacy of tositumomab.
We and others have shown that the PCD induced by a type II CD20 mAb is not classic apoptosis because it does not involve DNA fragmentation or caspase processing, it cannot be regulated by Bcl-2 and it lacks any of the hallmark ultrastructural features15 (reviewed by Cragg et al42 ). One obvious explanation for the in vivo potency of tositumomab reported here and elsewhere15 therefore is that it relates directly to the ability to induce nonapoptotic PCD. Unfortunately, attempts to date to demonstrate PCD in vivo after tositumomab treatment have been unsuccessful, leaving this suggestion as interesting speculation and the subject of ongoing research.
In conclusion, here we have presented evidence using 2 strains of hCD20 transgenic mice that a type II anti-CD20 mAb is significantly more effective than type I reagents at depleting normal B cells in vivo. Similar enhanced efficacy was recently reported for another type II mAb, GA101, under development by Umana et al.43 This enhanced efficacy is observed throughout the lymphoid organs and cannot be explained by differences in affinity, binding density, half-life, isotype, or complement activation, but is more probably to relate to other inherent properties of type II mAb, such as their ability to mediate PCD. These results provide strong support for the development of type II anti-CD20 mAbs for the treatment of B-cell diseases, particularly as we might expect that their lack of complement engagement should reduce the toxic side effects often associated with the use of rituximab and other type I reagents.9
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the CD20 research team, past and present, for their contributions, together with Prof Tim Illidge (Manchester, United Kingdom) and Dr Luke Nolan (Southampton, United Kingdom) for the supply of anti-CD20 mAb (tositumomab and rituximab), and Prof Marina Botto (London, United Kingdom) and Aras Kadioglu (Leicester, United Kingdom) for the provision of C1q−/− mice.
This work was supported by the Association for International Cancer Research (Fife, United Kingdom), Leukaemia Research Foundation (London, United Kingdom), and Tenovus (Cardiff, United Kingdom).
Authorship
Contribution: S.A.B. helped design the research, performed experiments, analyzed results, produced figures, and wrote the paper; R.R.F., S.J., K.E.A., and C.M.B. performed experiments; C.H.T.C. produced critical reagents; A.A. and M.J.S. generated and provided mice; M.J.G. designed the research, analyzed results, and edited the manuscript; M.S.C. performed experiments, designed the research, analyzed results, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin J. Glennie, Tenovus Laboratory, Cancer Sciences Division, Southampton University School of Medicine, General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: mjg@soton.ac.uk.
References
Author notes
*M.S.C. and M.J.G. are considered senior authors and contributed equally to the project.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal