Abstract
Ficolins are soluble pattern recognition molecules that bind carbohydrate structures on the surface of microorganisms. Three ficolins have been identified in man: ficolin-1, ficolin-2, and ficolin-3. Ficolin-1 is derived from the FCN1 gene on chromosome 9 and is synthesized in monocytes and type II alveolar epithelial cells. Ficolin-1 has been shown to be present in secretory granules of human neutrophils, but which subset of the neutrophils secretory granules harbors ficolin-1 is not known. In order to determine the exact subcellular localization of ficolin-1 in neutrophils, recombinant ficolin-1 was expressed in Chinese hamster ovary cells and used for generation of polyclonal antibodies. This allowed detection of ficolin-1 in subcellular fractions of human neutrophils by ELISA and western blotting, and by immunohistochemistry. Real time PCR examination of normal human bone marrow showed FCN1 gene expression largely in myelocytes, metamyelocytes, and band cells with a profile quite similar to that of gelatinase. In accordance with this, immunohistochemistry and subcellular fractionation demonstrated that ficolin-1 is primarily localized in gelatinase granules, but also in highly exocytosable gelatinase poor granules, not previously described.
Ficolin-1 is released from neutrophil granules by stimulation with fMLP or PMA, and a significant part becomes associated with the surface membrane of the cells and can be detected by flow cytometry. Our studies show that neutrophils are a major source of ficolin-1, which can be readily released to the surroundings by stimulation.
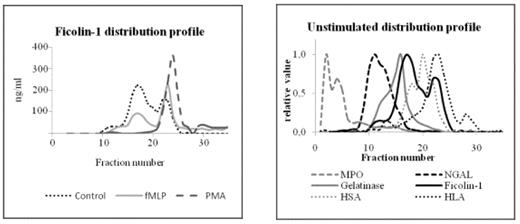
Left: Distribution profile of ficolin-1 in unstimulated (control) neutrophils and neutrophils stimulated with fMLP or PMA. Right: Distribution profile of ficolin-1 and granule marker proteins in subcellular fractions of unstimulated human neutrophils.
Left: Distribution profile of ficolin-1 in unstimulated (control) neutrophils and neutrophils stimulated with fMLP or PMA. Right: Distribution profile of ficolin-1 and granule marker proteins in subcellular fractions of unstimulated human neutrophils.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal