Abstract
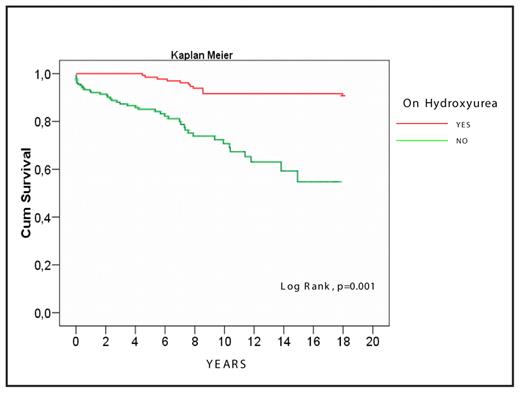
Hydroxycarbamide (hydroxyurea; HU) is now considered as the main pharmacological agent capable to prevent the painful crises, to reduce the frequency and length of hospital admissions, and to improve the quality of life of patients with sickle-cell disease (SCD); whether HU can prevent the severe chronic complications or modify the mortality of these patients remains still an interesting unresolved question. The present study aims to evaluate the above effects of HU in a large number of SCD patients who received HU over long period of time and were followed in a single Center. To this effect, we evaluated the records of 330 patients with SCD who have been followed in the Thalassemia Center of Laikon Hospital over the last twenty years (136M/194F; median age: 42 years, range: 20- 76 years); 34 with homozygous HbS (SS) and 296 compound heterozygotes for HbS and beta-thalassemia. Of the latter, 131 patients had HbS/beta0-thal and 165 HbS/beta+-thal (107 with the IVSI-110 and 58 with the IVSI-6 thalassemic mutation). Administration of HU was started as early as 1991; progressively, the total number of patients who received HU reached 131 (“group A”); the remaining 199 were treated conventionally (“group B”). The mean age of the patients in the two groups was similar (mean ±SD: 43.3±9.9 years for group A and 44.1±12.6 years for group B) as well as the levels of HbF. The usual dosage of HU was 20 mg/kg/day, except for some patients, where toxicity or lack of effectiveness imposed modifications of the dosage in the range of 15 to 35 mg/kg/day. The median follow-up period was 8 years (range: 0.1–17 years) for the patients who received HU and 5 years (range: 0.1–18 years) for those who did not receive HU. Apart rare exceptions, patients reported every 4–8 weeks to the Outpatient Clinic, where they had a thorough clinical and laboratory evaluation, including FBC and reticulocytes, LDH, bilirubin, other basic biochemistries and HbF using conventional techniques. The percentage of HbS/HbS, HbS/beta0-thal, and HbS/IVSI-110 patients who were given HU was 70%, 49% and 40% respectively; in contrast, very few HbS/IVSI-6 compound heterozygotes had symptoms justifying use of the drug (3.6%). Patients receiving HU showed a dramatic reduction of the frequency of severe painful crises (from 7.4±6.5 episodes per year pre-HU to 0.2±0.4 episodes post-HU; p<0.001) along with a significant reduction of transfusion requirements (mean number of administered packed red cell units: 1.5±5.9 per year pre-HU diminishing to almost zero during HU treatment; p<0.001). These results led to a significant reduction of hospital admissions from 2.1±2.9 per year pre-HU to 0.06±0.02 per year post-HU (p<0.001). Moreover, we noticed a significant reduction of the frequency of chest syndrome from 6.1% pre-HU to 0.8% during the HU period (p=0.013). In contrast, we failed to observe any significant differences in the frequency of aseptic avascular necrosis (p=0.219) or cerebrovascular episodes among the patients who received and those who did not receive HU (p=0.65). Importantly, the death rate in the group of patients receiving HU was significantly lower than that observed among patients who were conventionally treated (12 deaths in group A vs. 47 in group B; 9.1% vs. 23.6%, respectively; p=0.001). The probability of 10-year survival was 91% for the patients treated with HU, while that of the patients conventionally treated was only 70% (Figure). This difference is further magnified if one considers that prior to starting HU the patients of group A had significantly higher transfusion requirements, frequency of painful crises, yearly number of admissions to hospital and intensity of hemolysis in comparison to the group of patients who were not given HU (p=0.004, <0.001, <0.001, and 0.01, respectively). Factors that predicted for a superior survival in the HU group include the increased level of total hemoglobin, a reduced platelet and leukocyte count, increased HbF levels, low reticulocyte counts and low bilirubin and serum LDH levels at 6 months post-HU administration. Side-effects of HU therapy were minimal, predictable and easily manageable. Our study provides evidence that, in addition to reducing the number of painful crises, the frequency of hospital admissions and the transfusion requirements, the long-term administration of HU in adult patients with sickle cell syndromes can improve survival. These results highlight the beneficial effect of HU in SCD and raise the issue of expanding the use of HU in all patients with these conditions.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal