Abstract
BACKGROUND: In acute myeloid leukemia (AML), cytogenetic and molecular genetic abnormalities are known to play an essential role in the pathogenesis and are now accepted to be of paramount prognostic significance. However, mitochondrial dysfunction is also emerging as a major factor of importance in cancer. The mitochondrion has its own double-stranded circular 16.569 base pairs DNA (mtDNA) encoding 13 genes involved in oxidative phosphorylation and the respiratory chain, 2 rRNAs, and 22 tRNAs. As such, they are important in apoptosis and might, thus, be crucial in response to chemotherapy and to disease progression. The purpose of this study was to determine if mtDNA mutations are of importance to outcome of chemotherapy and to long-term survival in AML.
METHODS: The whole mitochondrial genome was sequenced using a resequencing system based on 46 PCR amplicons (MitoSEQr, Applied Biosystems, Foster City, CA) performed on a Genetic Analyzer 3130 (Applied Biosystems). Diagnostic bone marrow from 20 patients with AML, treated with curative intent, was analyzed. To avoid problems with misinterpretation of heteroplasmy due to admixture of other non-malignant cells, all patient samples selected had more than 80 percent blasts according to immunophenotyping. Data were analyzed using SeqScape v.2.5, Applied Biosystems and statistically analysis in Stata 10.
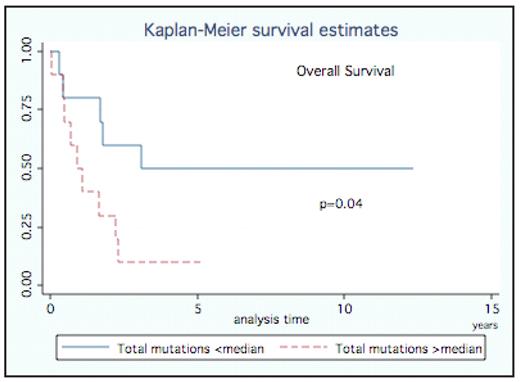
RESULTS: We sequenced the entire mitochondrial genome in 20 patients with AML with 99.5% base pairs sequenced (in 20 patients 329.734 base pairs were successfully sequenced out of 331.380 possible), and compared our findings with clinical data and survival data. In both coding and non-coding regions, a total number of 432 mutations (substitutions, insertions, and deletions) (range 8–44, median 15.5) were found. Mutations were scattered throughout the entire mitochondrial genome, and observed in all genes as well as in non-coding regions. Though, most were known polymorphisms in the Mitomap database (www.mitomap.org), eleven of the non-synonymous mutations were novel in the Mitomap database. All patients had non-synonymous mutations, resulting in amino acid changes (range 2–10, median 3.5), with a total number of 90 non-synonymous mutations. Two of the known non-synonymous mutations were present in all patients (A8860G, A15326G). While most changes were homoplasmic changes, heteroplasmic ones were observed in 12 of 20 patients (range 1-2). Notably, by dividing the patients by the median of the total number of mutations, patients with less than 16 mutations have a 5 years survival of 50% as compared to 10% for patients with 16 mutations or more. This revealed a significant (p=0.04) impact on overall survival of total number of mutations in both coding and non-coding regions (Fig.). Importantly, regression analysis revealed that the number of mutations was independent of age. The non-synonymous mutations show a trend towards a difference in overall survival (p=0.07).
CONCLUSION: This is, to our knowledge, the first demonstration of a prognostic impact on survival in AML patients of mutations in the mitochondrial DNA. Further studies on more patients are, however, clearly warranted to discern by which mechanisms mitochondrial DNA mutations are impacting prognosis in AML. Disclosure: No relevant conflict of interest to declare.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal