Abstract
Transferrin receptor 1 (TfR1 or CD71) is the major gateway for iron entry into cells and is overexpressed on a variety of cancer cells. Antibodies targeting TfR1 have shown efficacy in vitro and in vivo against different malignancies by affecting internalization of iron into target cells; however, in some cases their response rate is limited despite the high expression of TfR1 on the target cells. The cause of this limited response is still not well understood. We have developed a mouse/human chimeric IgG3-avidin fusion protein (ch128.1Av) that binds specifically to human TfR1 and exhibits a significant intrinsic anti-proliferative and pro-apoptotic activity against hematopoietic malignant cells by inducing TfR1 degradation and iron starvation. We have previously found a wide range of responses to treatment with ch128.1Av, which can be categorized as high, intermediate, or low sensitivity to the drug. Among the cells tested, the human B-lymphoblastoid cell line IM-9 was one of the most sensitive to ch128.1Av, while the human myeloma cell line U266 was tolerant. To identify the mechanisms responsible for mediating sensitivity or tolerance to the treatment with ch128.1Av, we conducted confocal microscopy studies and a whole genome time-course microarray analysis of IM-9 and U266 cells treated with this drug. In the current work we describe the molecular pathways associated with sensitivity or resistance to this therapy in vitro. We demonstrate that IM-9 cells treated with ch128.1Av can re-route TfR into the lysosomal compartment and exhibit a cytotoxic response that is predicted to be mediated in part by p53 and its target genes based on a transcription factor binding site analysis of the promoter region of genes affected by treatment with ch128.1Av. We show that a siRNA mediated reduction of wild type p53 expression rescues in part the inhibition of proliferation of IM-9 cells treated with ch128.1Av, which validates the in silico promoter analysis. In contrast, we show that U266 cells are tolerant to the cytotoxic response induced by ch128.1Av, probably due to a combination of factors including their failure to redirect trafficking of TfR targeted by ch128.1Av into the lysosomal compartment, lack of a wild type copy of the p53 protein, an early pro-proliferative response to treatment, and a high expression of the cyclin D1 gene. This work has important implications in the clinical use of TfR targeted therapies and is broadly applicable to the advancement and development of novel therapeutics targeting iron metabolism in hematopoietic malignancies.
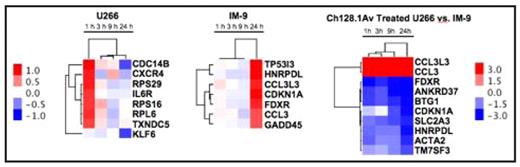
Hierarchical clustering of expression changes (p<0.05) in ch128.1Av treated IM-9 and U266 cells, or between the two cell lines sampled at 1 h, 3 h, 9 h, and 24 h post treatment. The hierarchical clustering of genes and treatment times is based on their expression changes and determined by an un-centered correlation algorithm. Genes shown are also those with the greatest time-dependent variance in ch128.1Av treated IM-9 and U266 cells compared to their time-matched controls. Color scale-bar included shows fold changes in gene expression in log base 2 scale.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal