Abstract
CD34 positive cells from patients with trisomy 8 myelodysplastic syndrome (MDS) have pronounced expression of early apoptotic markers compared to normal hematopoietic cells. However, trisomy 8 clones persist in patients with bone marrow failure and expand following immunosuppression (
Using fluorescent in situ hybridization (FISH) we showed that the novel styryl sulfone, ON 01910.Na (
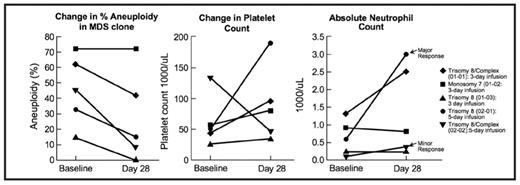
These encouraging in vitro data led to a phase I/II trial of ON 01910.Na in MDS patients with refractory anemia with excess blasts who had IPSS =/> int-2. This study was designed to assess the safety, and activity of escalating doses of ON 01910.Na (800 mg/m2/day × 3 days, 800 mg/m2/day × 5 days, 1500 mg/m2/day × 5 days, 1800 mg/m2/day × 5 days every 2 weeks) in MDS patients. To date five MDS patients have been treated with ON 01910.Na for 4 to 16 weeks in the first two dose cohorts. Two patients had isolated trisomy 8, two had complex cytogenetic abnormalities including trisomy 8 in all aneuploid cells, and one had monosomy 7. Three and five day infusions were well tolerated. Pharmakokinetic analysis showed that the half life of the drug is 1.3 ± 0.5 hours without signs of drug accumulation. Four of five patients demonstrated a rapid and significant decrease in the number of peripheral blasts and aneuploid cells after 4 weeks of therapy (see below), concomitantly with increases in neutrophil and/or platelet counts in four patients. All four patients exhibiting a biological effect of drug treatment had trisomy 8 in their aneuploid clone prior to therapy. One monosomy 7 patient, previously refractory to EPO became responsive to Darbopoietin and another trisomy 8 patient became platelet-transfusion independent.
In this early safety trial, ON 01910.Na demonstrates efficacy at early timepoints with respect to improved cytopenias and decreased blast counts. Continued enrollment and long term follow-up will further detail clinical efficacy and impact on the long term prognosis of high risk MDS patients treat with this drug.
Disclosures: Wilhelm:Onconova Therapeutics: Employment. Maniar:Onconova Therapeutics: Employment.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal