Abstract
Dendritic cell (DC) vaccines in leukaemia show promise as a novel treatment modality aimed at reducing relapse rates by augmenting immune responses when patients are in a state of minimal residual disease. DC vaccination studies to date have not been as successful as hoped and this may be because of insufficient cytotoxic T cell (CTL) responses. Immunomodulatory agents such as 4-IBB ligand, and cytokines such as IL2, IL6 and GM-CSF have been reported to improve vaccine therapy however these agents have to be tolerated clinically and this limits the use of some preparations. Our study aims to generate a more efficient DC vaccine process by using Lenalidomide, a thalidomide analogue and immunomodulatory agent which is a powerful potentiator of CTLs and natural killer cells. It has the advantage of clinical tolerability and is already in use for treating haematological malignancies.
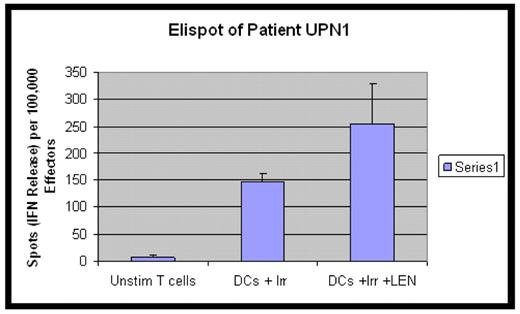
In this in vitro study DCs were generated from monocytes of eight patients in remission following chemotherapy for acute myeloid leukaemia (AML) or chronic myeloid leukaemia (CML) with standard cytokine protocols. The immature DCs were loaded with autologous leukaemia cells from the patients’ presentation samples. The presentation leukaemia cells were treated with UVB irradiation to induce apoptosis. DCs were matured with TNF alpha for two days then co-cultured with autologous T cells for one week with or without the addition of Lenalidomide at ten micromolar. A control group of unstimulated T cells were kept in culture for the same time period. The T cells from the three groups were harvested and their cytoxicity assessed in an Interferon Gamma (IFNγ) ELISPOT assay where the stimulators used were the unmodified blasts. Results in all eight patients show the leukaemia specific CTL responses (as measured by IFNγ release) were markedly improved in the DC/irradiated blasts groups compared with the unstimulated T cells. In six out of eight patients there was a further, significant improvement in the groups where Lenalidomide was added (see Figure 1).
These results show promise for the use Lenalidomide as an agent to optimise CTL responses to DC based Immunotherapy for myeloid leukaemia and should be investigated further in a clinical trial.
Disclosures: Off Label Use: Lenalidomide is being used as an Immunomodulatory agent within a Dendritic Cell Vaccination pre-clinical study. As a conclusion within the abstract we suggest going on to use it in a clinical study..
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal