Abstract
The CLL4 trial evaluated 1st line treatment with F or FC among 375 CLL patients up to 65 years (Eichhorst et al., Blood, 2006). Updated follow-up (median 52.8 months (mo) for alive patients) revealed 50.4% events for progression-free survival (PFS) and 24.5% for overall survival (OS). Comparing the treatment arms, significant differences were observed in favor of FC for CR (19.9% vs 5.9%, p<.001), CR+PR (94.7% vs 82.4%, p<.001), and PFS (median 67.8 vs 43.7 mo, p=.002) but not for OS (median n.r. vs 84.6 mo, p=.191).
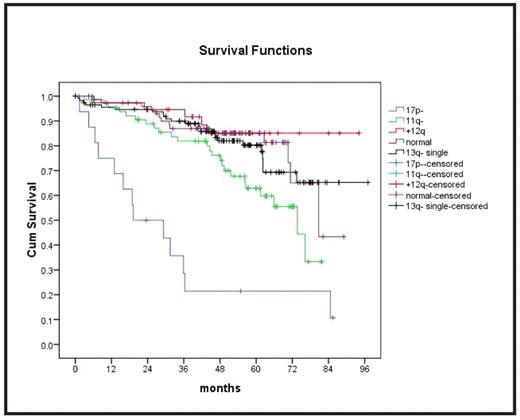
The incidences of genomic aberrations by FISH (n=321) were 13q- 50.5%, 13q- single 36.0%, 11q- 21.1%, +12 13.6%, and 17p- 5.0%. No aberration was found in 25.9%. TP53 mutations (n=340) were observed in 8.8%. VH status (n=340) was unmutated in 64.3% and V3–21 was rearranged in 5.9%. ZAP70 (n=236) was >20% in 37.7% and CD38 (n=259) was >7% in 47.9%. β2-MG and TK (both n=261) were >5 mg/L in 13.8% and > 10U/L in 73.0%, respectively. The distributions of these biologic markers and the major clinical characteristics (age: median 59 (42–65) years, sex: 73.1% male, stage: Binet A 9.2%, B 55.4%, C 35.4%, LDH > 250U/L: 39.2%, lymphocytes > 50/nl: 57.1%, comorbidities >1: 21.4%, and creatinine clearance were not significantly different between treatment arms. Outcome was analyzed for subgroups defined by the clinical and biologic parameters in univariate analyses for the entire population (i.e. both treatment arms combined). PFS was significantly shorter for 11q-, 17p- (Fig.1), TP53 mutation, elevated TK and LDH. OS was significantly shorter for the same parameters and unmutated VH, elevated β2-MG, and >1 comorbidities.
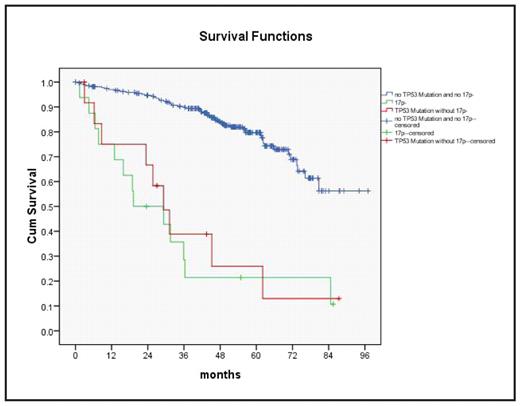
All except 2 patients with 17p- had TP53 mutations. There were 13 additional cases with TP53 mutation without 17p-. OS was similarly poor for TP53 mutation without 17p- as compared to 17p- and all other cases (OS at 48 mo: 25.9%, 21.4%, and 84.6%, respectively, p<.001) (Fig. 2). To investigate specific treatment effects, the efficacy of F and FC was compared in subgroups defined by biologic markers. PFS was longer after FC only for the subgroups with unmutated VH (p=.005), no aberration (p=.010), 11q- (p=.017), unmutated TP53 (p=.001), CD38 >7% (p=.011), and β2MG <5mg/L (p=.009). OS was significantly longer after FC only in the subgroups with 11q- (p=.037), +12 (p=.044), and unmutated TP53 (p=.029).
Multivariate analysis was performed by Cox regression with backward selection including all clinical and biologic parameters in addition to the treatment arms. Regarding PFS, independent prognostic factors were FC (HR 0.68, p=.047), TK (HR 1.62, p=.051), 11q- (HR 1.66, p=.015), and TP53 mutation (HR 4.16, p<.001). Similarly, for OS independent factors were FC (HR 0.56, p=.033), TK (HR 2.79, p=.009), 11q- (HR 1.98, p=.015), and TP53 mutation (HR 11.61, p<.001). If TP53 mutation was not included in the analyses, 17p- entered the model as significant factor (PFS: HR 3.60, p=.003, OS: HR 12.64, p<.001).
In summary, biologic parameters are the major determinants of outcome after 1st line treatment with F and FC in CLL. The overall improvement after FC as compared to F appears to result from specific treatment effects in distinct biologic subgroups. However, in comprehensive multivariate analysis, TP53 mutation, 11q-, and TK > 10 remain predictors for PFS and OS independently of the improvement by FC. TP53 mutation and 17p-, although largely overlapping, deserve further evaluation for their independent impact.
Disclosures: Stilgenbauer:Bayer: Consultancy, Honoraria, Research Funding. Off Label Use: F and FC 1st line in CLL.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal