Abstract
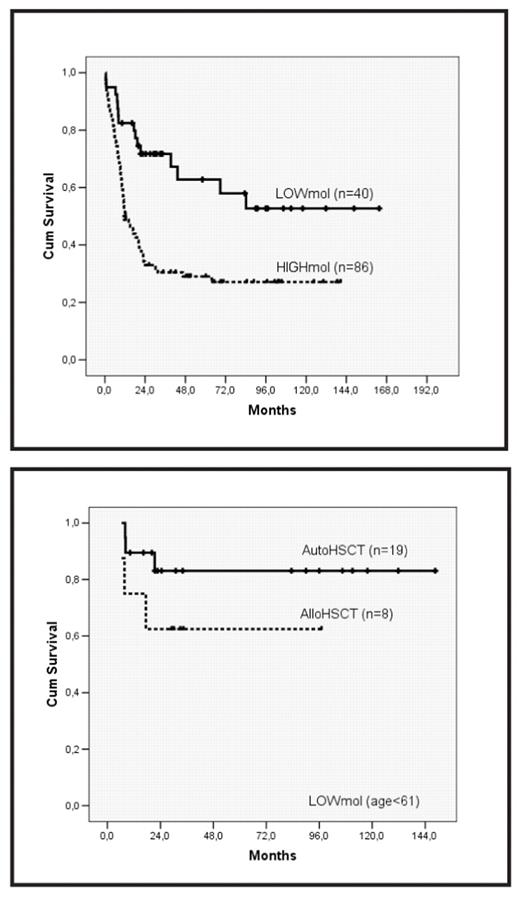
The heterogeneous prognosis of patients with intermediate-risk cytogenetics AML (AML-IR) can be partially clarified by screening of NPM1 mutations (NPMmut) and internal tandem duplication of FLT3 (FLT3-ITD). Nonetheless, additional factors might influence the prognostic effect of these molecular lesions, such as the FLT3-ITD mutant level. Moreover, the optimal post-remission strategy might differ depending on the underlying molecular lesion. In this regard, we analyzed the outcome, according to NPM1 and FLT3 mutations and post-remission therapy given, of a series of patients diagnosed with de novo AML-IR in a single institution who received intensive chemotherapy. Patients were treated following 4 sequential protocols of CETLAM group during the period 1994–2006, consisting of 1 or 2 cycles of standard induction chemotherapy and 1 course of high-dose cytarabine-based consolidation therapy. Thereafter, patients underwent hematopoietic stem cell transplantation (HSCT) according to donor availability and presumed risk (protocols LAM 99 & 2003). NPM1 mutations and FLT3-ITD were screened in diagnostic DNA by PCR amplification followed by Genescan analysis. The ratio between FLT3-ITD and wildtype FLT3 alleles (ITD/wt ratio) was calculated using the area under the peak of corresponding alleles. Overall, 134 patients (51% male; median age, 53; range: 17–70) with AML-IR (normal karyotype, 66%) were studied. NPM1mut and FLT3-ITD were found in 45% and 37% of patients, respectively, with a median ITD/wt ratio of 0.59 (0.045–5.5). After induction regimen, 109 patients (81%) achieved complete response (CR). The only variables predictive of a favorable response were NPMmut (90% vs. 75%; p=0.01) and age <60 (85% vs. 72.5%; p=0.05). After a median follow-up of 69 months, relapse risk (RR) at 5 years was 54% (±5%). RR was higher in patients presenting with hyperleukocytosis (>50 × 109/L), NPMwt, and FLT3-ITD. Interestingly, the prognostic value of FLT3-ITD depended on the relative mutant level, and detection of FLT3-ITD with a low ITD/wt ratio (i.e.,<0.3) did not increase the risk conferred by underlying NPM1 mutational status. In accordance, a composite variable based on NPMmut and quantitative FLT3-ITD was created defining 2 prognostic categories: a low-risk group (LOWmol), constituted by patients with NPMmut and either absence of FLT3-ITD or low ITD/wt ratio, and a high-risk subset (HIGHmol), defined by the absence of NPMmut and/or high ITD/wt ratio. This molecular stratification showed independent prognostic value for RR (5-year RR: 24%±10% vs. 81%±7 in LOWmol vs. HIGHmol patients, respectively; p<0.001), and survival (OS; relative risk: 2.8, 95% CI:1.6-5, p<0.001; figure
Moreover, the effect of post-remission therapy varied in both molecular-defined subgroups. Thus, among patients with an age ≤60, 5-year survival in LOWmol patients with a planned autologous HSCT (autoHSCT) was 83%±9%, not differing significantly from that of patients undergoing allogeneic HSCT (intention-to-treat analysis; figure
On the other hand, 5-year OS of HIGHmol patients who underwent allogeneic HSCT in first CR was 73%±13, which compared favorably with other post-remission strategies (5-yr OS: 27%±7%; p=0.019).
In conclusion, in patients with intermediate-risk AML, the combined assessment of NPM1 mutations and quantitative estimation of FLT3-ITD allows the distinction of 2 categories of patients with different prognosis. Thus, whereas autoHSCT arises as an effective postremission therapy in patients harboring low-risk molecular features, allogeneic HSCT in first CR seems to overcome adverse prognosis of patients with high-risk disease.
Nonetheless, the validity of this molecularly-based therapeutic algorithm warrants confirmation in other studies.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal