Abstract
Low risk clonal myelodysplastic syndromes (MDS) (blast count less <5%, without ring sideroblasts and a normal karyotype) can be difficult to differentiate morphologically from dysplastic non-clonal disorders. Both cause clinically significant cytopenias in the elderly and are increasing in prevalence in an ageing population. Flow cytometry has been used to try and distinguish MDS from non-clonal cytopenias. However, no one simple flow-based marker reliably differentiates MDS from non-clonal cytopenias. This has given rise to a number of scoring systems combining multiple measurements. They have variable sensitivity/specificity in MDS diagnosis and can be complex, which detracts from their routine use in clinical non-specialist flow cytometry laboratories. Perhaps the most common abnormality that we, and others, have documented in MDS is reduced B-cell CD34+ progenitors. In addition, high risk MDS is associated with increased immature hematopoietic precursors/progenitors. As immature haematopoietic precursors have low CD38 expression whereas CD34+ B-cell progenitors express high CD38 levels, we hypothesised that the relative mean fluorescence intensity (RMFI) of CD38 expression on CD34+ cells would be decreased in MDS and could provide a simple useful single flow cytometric measurement diagnostic of MDS. We initially compared CD34+ cells from low-grade MDS patients (n=10), normal controls (n=5) and pathological controls with non-clonal cytopenias (n=10) (Figure 1). High numbers of nucleated bone marrow cells (>0.5×106) were acquired from each sample to provide an accurate analysis CD38 RMFI in low frequency CD34+ subsets. CD38 RMFI in CD34+ cells where lower in MDS patients (mean=27.6; range: 5.6–51.6) than normal controls (mean=58; range: 33.3–78.8) and, importantly, there was a more striking reduction and no overlap in the range of values, when compared to the pathological controls (mean=194.5; range: 60.9–573). We then studied a second prospective cohort of bone marrow samples from 90 MDS patients and, as control samples, 31 cases of acute myeloid leukaemia, 11 of chronic myelomonocytic leukaemia and mixed MDS and myeloproliferative disorder and 70 of non-clonal cytopenias in a independent laboratory that routinely provides clinical diagnostic service. Remarkably, a reduced threshold value of CD38 conjugated with phycoerthyrin (PE) RMFI as defined by receiver-operator characteristic (ROC) curve diagnosed low-grade MDS with 95% sensitivity (95% confidence interval, 87–99%) and 92% specificity (95% confidence interval, 82–97%) (Figure 2–the dotted line is the threshold value derived from the ROC curve). This was due principally to both a decreased number of CD34+ B-cell progenitors and an increase in immature CD34+ immature precursors (Figure 2B). CD34+ cells from RAEB patients displayed an even greater reduction in CD38-PE RMFI linked to an increased frequency of CD34+CD38lo/− progenitors (Figure 1B). Similar results were seen in AML samples (Figure 2). In conclusion, reduced CD38-PE RMFI on CD34+ cells is a simple single flow cytometric test that may be of value in the routine clinical diagnosis of MDS, especially cases with a low blast count and normal karyotype.
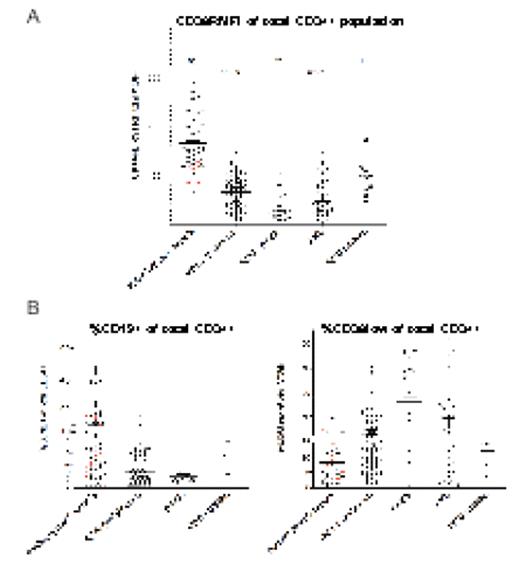
(A), CD38RFMI in CD34+ cells in the first group of samples studied. (B), The percentage of CD34+CD19+ and CD34+CD38lo cells in the samples studied in (A)
(A), CD38RFMI in CD34+ cells in the first group of samples studied. (B), The percentage of CD34+CD19+ and CD34+CD38lo cells in the samples studied in (A)
(A), CD38RFMI in CD34+ cells in the second group of samples studied. Samples in red are from patients with autoimmune cytopenias. (B), The percentage of CD34+CD19 and CD34+CD38lo cells in the samples studied in (A)..
(A), CD38RFMI in CD34+ cells in the second group of samples studied. Samples in red are from patients with autoimmune cytopenias. (B), The percentage of CD34+CD19 and CD34+CD38lo cells in the samples studied in (A)..
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal