Abstract
Aim: Pre-clinical data suggest that lenalidomide imparts an immunomodulatory effect. This clinical trial in relapsed myeloma patients examined the ability of lenalidomide to augment both endogenous as well as vaccine-specific immune responses in vivo.
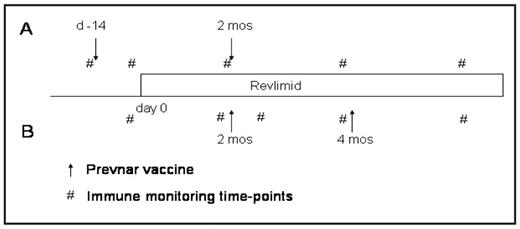
Methods: Relapsed, lenalidomide naïve, patients treated with 3 or less prior regimens were eligible for the study. Prevnar®, a pneumococcal vaccine, was given either before or during administration of lenalidomide in two cohorts of patients. Cohort A received their first vaccination prior to administration of drug, and the second vaccine on cycle 2, day 15 of lenalidomide. Cohort B were first vaccinated on cycle 2, day 15 and then cycle 4, day 15. Patients were treated with 25mg of lenalidomide daily days 1–21 every 28 days for 6 cycles. Pneumococcal serotype titres as well as CRM-197 T cell responses quantified the B and T cell responses, respectively, to Prevnar vaccination and were correlated with lenalidomide administration. Systemic immune responsiveness was determined by delayed type hypersensitivity (DTH) responses to Candida and tetanus and quantification of cytokines in the peripheral blood (PBL) serum and bone marrow (BM) plasma.
Results: A median two-fold increase in antibody responses to Prevnar was observed in cohort B, whereas cohort A demonstrated an 80% decrease in antibody titres. Antibody responses in the bone marrow were more pronounced than in blood and were greatest in Cohort B. 1.8% of the total T cell population proliferated to CRM-197 in Cohort B vs. 0% in Cohort A. Increases in DTH responses were seen in 50% of patients post lenalidomide. Luminex was utilized to measure cytokine levels pre and post lenalidomide. Globally, IL-6 levels were greatly reduced in both the BM (88% reduction) and PBL (77% reduction) samples. Both IFNγ and IL-17 were undetectable in the PBL samples, but were elevated and unchanged respectively in BM samples. Levels of IL-10 peaked in both cohorts after the first vaccination but were ultimately reduced with the administration of lenalidomide, and overall the levels were higher in the BM than PBL samples. MCP-1 and MIP-1β levels showed an overall decrease over the course of the trial. There was no alteration of IL2, IL-4, IL-5, TNFα, IL-7, IL-1 β, IL-12, IL-13, G-CSF or GM-CSF levels with the administration of lenalidomide.
Conclusions: This is the first comprehensive examination of the immunomodulatory effect of lenalidomide on global and vaccine specific in vivo immune responses. We show that the most potent immune response was observed when both prime and boost vaccines were administered while receiving lenalidomide. Immune enhancement by lenalidomide was seen in both the blood and BM compartments. Of note, the serologic titres were greater in the BM than blood and the T cell responses (when observed) appeared greater in the BM. These data provide evidence of the important role of bone marrow niche in the maintenance of immune memory responses. The increased DTH response to both Candida and tetanus provides in vivo evidence of lenalidomide-mediated immune enhancement. Taken together, these data demonstrate that lenalidomide augments in vivo immune responses in patients with advanced/relapsed multiple myeloma. This study provides the rationale for utilizing this drug in combination with cancer vaccines to augment anti-tumor efficacy or with infectious vaccines.
Disclosures: Knight:Celgene: Employment. Borrello:Celgene: Research Funding, Speakers Bureau. Off Label Use: The use of lenalidomide as an immunomodulatory drug..
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal