Abstract
Background: Secondary acute myeloid leukemia (sAML), evolving from prior MDS or leukemogenic therapy (tAML), is associated with a poor prognosis. Many sAML patients are elderly with significant comorbidities. The incidence of unfavorable cytogenetics and expression of multidrug resistance (MDR) phenotype is higher in sAML blasts. Amonafide (AS1413) is a DNA intercalating agent and unique topoisomerase II (Topo II) inhibitor that, unlike the classical Topo II inhibitors (DNR, IDA, mitoxantrone, etoposide), does not affect the DNA/Topo II cleavable complex that results in DNA fragmentation. It does not act as either a substrate or an inhibitor of the MDR efflux pump, P-glycoprotein (Pgp), nor is it cross-resistant with classical Topo II inhibitors. A complete remission (CR) rate of 38.6%, with an additional 3.4% of patients achieving CRp, has previously been reported for this phase II trial. Updated findings from this trial including duration of remission data are reported here.
Methods: Patients received a 4 hr infusion of amonafide 600 mg/m2/day × 5 and continuous IV infusion cytarabine 200 mg/m2/day × 7. 14 patients received a second course on day 14 for persistent leukemia. Patients received consolidation with stem cell transplant or intermediate/high dose cytarabine, depending on age. Central bone marrow review was performed for patients in CR. The primary endpoint was CR + CRp. Secondary endpoints included duration of CR/CRp. The last patient was treated in December 2006.
Results: 88 patients were enrolled; median age was 62.5 (range 23–87); prior MDS, 48.9%, (43/88); tAML, 51.1% (45/88); unfavourable cytogenetics, 46.6% (41/88). The most common non-hematologic grade ≥ 3 AEs seen were febrile neutropenia (35.2%), hypotension (15.9%), pneumonia (14.8%), hypokalemia (12.5%), and bacteremia (11.4%). Death rate within 28 days was 20.5%. Overall CR + CRp rate: 42.0% (37/88), including 38.6% (34/88) CR; 3.4% (3/88) CRp. CR by age < 60: 39.4% (13/33); ≥ 60: 43.6% (24/55). 30/37 patients received post-remission therapy with BMT (7), high/intermediate dose cytarabine (21) or both (2).
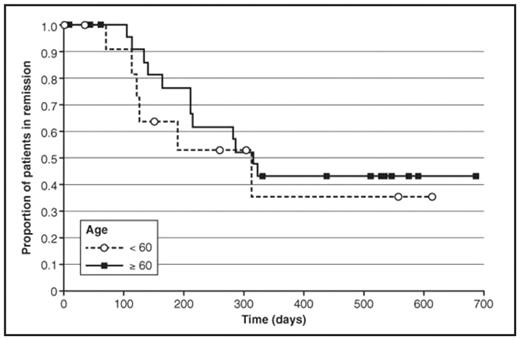
Kaplan-Meier estimate of the proportion of responders in continuous CR/CRp at 18 months is 41% for all sAML patients, and is similar for all poor-risk subgroups including age ≥ 60, 43%; tAML, 57%; and unfavorable cytogenetics, 38%. Median overall survival is 209 days for all patients and 400 days for patients with CR/CRp.
Conclusions: Durable CRs have been achieved in patients with sAML/tAML treated with amonafide plus cytarabine. This efficacy was similar across subgroups independent of age or the presence of various poor risk characteristics. Phase III evaluation of amonafide plus cytarabine vs. daunorubicin plus cytarabine (3+7) is currently underway.
Duration of Remission
Disclosures: Allen:Antisoma: Research Funding. Erba:Antisoma: Research Funding. Rizzieri:Antisoma: Research Funding. O’Donnell:Antisoma: Research Funding. Powell:Antisoma: Research Funding. Lundberg:Antisoma: Employment. Bennett:Antisoma: Research Funding. Capizzi:Antisoma: Employment.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal