Abstract
Immune-based therapies may improve outcome for multiple myeloma (MM) by eradicating chemo-resistant disease. Our recent trial utilizing IL2 activated, killer immunoglobulin-like receptor-ligand mismatched NK cell transfusions from haplo-identical donors yielded (n) CR in 50% of patients. Unfortunately, after NK cell therapy, 2/10 patients had progressive disease, and the median duration of response for the other 8/10 patients was only 105 days (range 58–593). This may have been due to an insufficient dose of alloreactive NK cells and early rejection. Furthermore, appropriate donors were identified for only 30% of otherwise eligible patients. We therefore investigated whether NK cells from MM patients could be expanded and activated to kill autologous MM. We then examined whether pre-treatment of MM cell targets with elotuzumab, a humanized antibody to the MM tumor antigen CS1, could further enhance NK cell-mediated lysis.
PBMC from 5 MM patients were co-cultured for 14 days with irradiated K562 cells transfected with 4-1BBL and membrane bound IL15 in the presence of IL2 (300U/ml) as previously described (
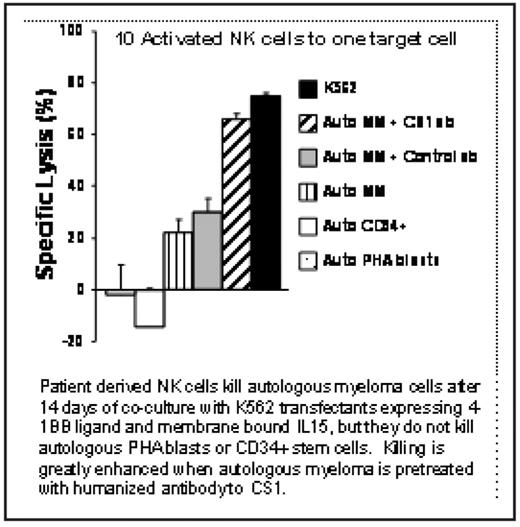
There was an average 64-fold expansion of NK cells (range: 8–200) after 2 weeks of co-culture with K562 transfectants. Expansion of T cells was not observed. The NK cell activating receptor NKG2D, and natural cytotoxicity receptors NKp30, NKp44, and NKp46 were up-regulated following the expansion. Expanded NK cells were able to kill autologous MM (E:T ratio 10:1, average 31%, range 22–41%), whereas resting NK cells did not. Pretreatment of autologous MM cells with elotuzumab increased the activated NK cell-mediated killing by 1.7-fold over target cells pretreated with an isotype control antibody. This level of killing was similar to that of the highly NK kill-sensitive cell line K562 (Figure). Autologous PHA blasts and CD34+ stem cells were not killed. Activated human NK cells were detectable in the bone marrow of NOD-SCID mice 48 hours after injection.
Ex vivo activation of NK cells from MM patients with K562 transfectants can induce killing of autologous MM and produce large numbers of NK cells for potential therapy. The addition of elotuzumab to activated NK cell therapy enhances anti-MM effects by ADCC thus invoking an additional NK cell-mediated mechanism of MM killing. Importantly, ex vivo activated NK cells traffic to the bone marrow in mice. Autologous NK cell therapy eliminates the issues related to allo-donor availability and early NK cell rejection, and could provide an option for patients refractory to chemotherapy agents.
Disclosures: Afar:PDL BioPharma: Employment. van Rhee:PDL BioPharma: Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal