Abstract
Background: The optimal therapy for patients with relapsed/refractory multiple myeloma remains controversial. Lenalidomide, an oral immunomodulatory drug, has been shown to be an effective treatment in this setting, with in-vitro laboratory data suggesting that its effect is synergistic with a number of established chemotherapeutic agents. The primary objective of this study was to determine the maximum tolerated dose and toxicity of cyclophosphamide when used in combination with lenalidomide and dexamethasone for patients with relapsed/refractory myeloma. The secondary objective was to determine clinical response rates.
Methods: Cyclophosphamide was given at increasing doses from 300 mg po in cohort 1, up to the maximum planned dose of 700 mg po in cohort 5 on days 1 and 8 of a 28 day cycle, in combination with dexamethasone (20mg po, daily on days 1–4 and 8–11) and Lenalidomide (25mg po, daily on days 1–21). Maximum tolerated dose (MTD) was established as one dose level below that at which 2 or more patients suffered dose limiting toxicity (DLT). DLT was defined as Grade 4 haematological toxicity or febrile neutropenia occurring during cycle 1 of treatment, or Grade 3/4 non-haematological toxicity during cycle 1, or a failure to start cycle 2 within 7 days of scheduled day due to toxicity. Once the MTD had been established, a further ten patients were enrolled at that dose. Safety assessments were performed weekly during cycle 1, and at the end of every cycle thereafter. Response data was collected on day 28 of each cycle.
Results:Toxicity The most commonly observed adverse events were; neutropenia (grade 1–3), cramps (grade 1–2), somnolence (grade 1–2), constipation/diarrhoea (grade 1–2), minor infections (grade 1–2), and musculoskeletal aches and pains (grade 1). The most common reason for dosing delays and dose reductions was grade 3 neutropenia.
Maximum tolerated dose No patients receiving doses of 300 to 600mg cyclophosphamide have experienced dose limiting toxicity during the first month of treatment. 2 patients receiving 700mg qualified as having dose limiting toxicity (One patient with febrile neutropenia, pneumonia and syncope (all grade 3)), and another with grade 4 pancytopenia). The maximum tolerated dose of cyclophosphamide in combination with lenalidomide and dexamethasone has therefore been established at 600mg daily on days 1 and 8 of a 28 day cycle.
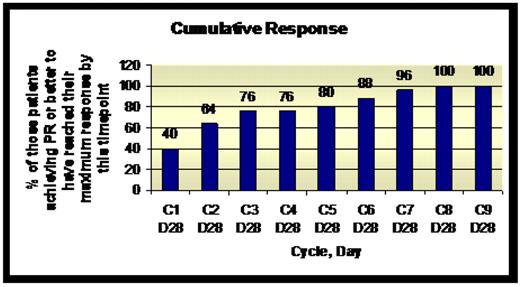
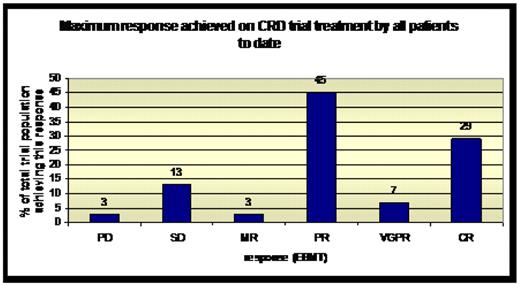
Response: A total of 31 patients were enrolled into the study. Median age at enrolment was 57 years (range 42–79). Median ISS at trial entry was 1 (range 1–3). Median time since last therapy was 7 months (range 1–84). To date, 36% of patients have achieved CR or VGPR, and 81% PR or better. Responses were assessed at the end of each cycle. 25 of the 31 patients have so far achieved PR or better. Of these 25 patients, 76% had achieved their maximum response by cycle 3 day 28. Patients had received 1–6 prior lines of therapy (median 3). 28/31 patients had previously received thalidomide, 20 had received autologous stem cell transplant, and 8 received velcade. 7 patients had received all 3 and, of these, 1 achieved SD, 3 PR, and 3 CR. This suggests that lenalidomide in combination with cyclophosphamide and dexamethasone is an effective therapy for heavily pre-treated patients.
Conclusion: 600mg cyclophosphamide on days 1 and 8 of a 28 day cycle is well tolerated in combination with Lenalidomide and dexamethasone. The combination is a highly effective therapy for relapsed/refractory myeloma. The majority of patients achieved a maximal response during the first 4 courses of treatment. CRD is also an effective treatment for patients who have received extensive prior chemotherapy. Whether patients need to continue treatment to nine cycles remains to be tested.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal