Abstract
Background: TRKA (Tropomyosin Receptor Kinase A) is a member of the neurotrophin tyrosine kinase receptor family. Nerve Growth Factor (NGF) binds specifically to TRKA triggering multiple downstream pathways which regulate proliferation and differentiation in a number of neural, epithelial and epidermal cell types. TRKA is also expressed in hematopoietic progenitor cells and NGF has been demonstrated to promote the colony growth and differentiation of myeloid progenitor cells. Interestingly, Pralle et al. found that 44% of patients with acute myeloid leukemia (AML) express TRKA mRNA and up to 30% encode activating TRKA mutations. Furthermore, a constitutively-active TRKA variant has been shown to contribute to myeloid leukemogenesis.
Purpose: To evaluate the role of NGF/TRKA signaling on AML proliferation and survival through effects on downstream signaling pathways, and evaluate the effects of TRK inhibition on human AML.
Methods: TRKA protein expression was measured in a panel of human AML cell lines (U937, K562, THP1, NB4, HL60, KU812). AlamarBlue (MTT) assays were used to measure proliferation/viability. For proliferation assays, cells were stimulated with NGF (5 ng/ml) for 1–3 days with or without the addition of a TRK inhibitor AZ-23 (25nM). Cell counts, cell cycle and survival were measured using trypan blue cell counts, and propidium iodine-based flow cytometry assays. Downstream signaling events were measured following a 15 minute exposure to recombinant human NGF. Antibodies for phosphorylated and total AKT, ERK, JNK and MEKK were used. In a human AML xenograft model, mice were treated with 100 mg/kg (2.5 mg) of AZ-23 by gavage for 2 weeks without toxicity. Weekly tail vein blood collection and flow cytometry were used to evaluate leukemia tumor burden. Kaplan-Meier curves were used to assess effects on morbidity.
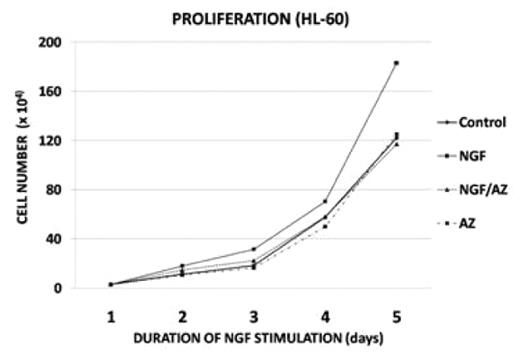
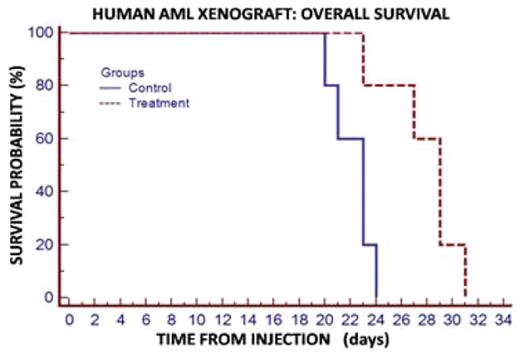
Results: Five of six human AML cell lines showed detectable TRKA protein levels by Western. When stimulated with NGF, all five cell lines which expressed TRKA demonstrated enhanced proliferation within 24 hours with mean value of 20% (10 to 45%, p=<0.001), which was abrogated with 25nM AZ-23 (p<0.01). The HL60 cell line showed the most impressive response to NGF (fig 1). The AML cell line which did not express TRKA (U937) and a human pre-B ALL cell line (Nalm-6) did not respond to NGF stimulation, as expected. There were no significant difference between only AZ-23 and the control group (p=>0.5). Following starvation, NGF stimulation induced the phosphorylation of ERK and AKT (2–4 fold), while inhibiting the phosphorylation of MEKK and JNK. All of these signaling effects were blocked with addition of AZ-23. Importantly, human AML-engrafted mice treated with the TRKA inhibitor AZ-23 had a significant decreased AML burden at 3 weeks post-inoculation (70% vs. 18% p=0.004). Kaplan-Meier estimates showed a Hazard Ratio of 10.5 (p=0.01) fig 3.
Conclusions: TRKA has been implicated in the proliferation and transformation of myeloid progenitors and may be expressed and activated in a significant subset of AML cases. Our studies provide evidence of an important role for NGF/TRKA signaling in human AML. We show that NGF induces TRK-dependent proliferation while inducing ERK and AKT phosphorylation, consistent with a proliferative and pro-survival effect. Furthermore, we show that NGF stimulation inhibits the phosphorylation of MEKK and JNK, which are associated with NGF withdrawal and promote apoptosis. Finally, we demonstrate in a human AML xenograft model that a selective TRK inhibitor AZ-23 can effectively reduce AML burden and enhance survival. Based on these results, we propose that TRK inhibition may be a therapeutic option for a subgroup of AML, and warrants further pre-clinical development.
Disclosures: Pien:Astrazeneca: Employment. Brown:Astrazeneca: Employment.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal