Abstract
Although allogeneic HSCT is potentially curative in myeloma, series reporting encouraging results with submyeloablative regimens suffer from limited follow-up and incomplete information on prognostic factors known to be relevant in myeloma. The outcome of 24 myeloma patients (50–66 years, median 52) undergoing reduced-intensity allogeneic HSCT after 100 mg/m2 melphalan from related (n=21) or unrelated (n=3) donors. The 9 patients who had not relapsed after preceding autotransplantation received 50 mg/kg cyclophosphamide in addition. No patient underwent a planned sequential autograft-allograft procedure.
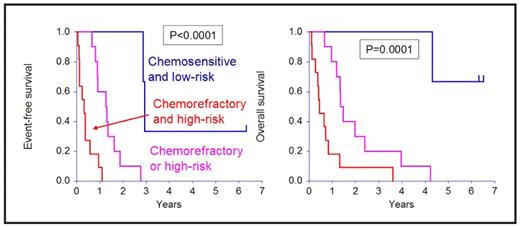
Complete prognostic factor information was available on each patient. High-risk disease (n=16) was defined by one or more of the following: del(13) on conventional karyotyping, t(4;14), del(17p), plasma cell labeling index >1%, elevated LDH, plasmablastic morphology. The disease was refractory to therapy in 16 patients. Three patients (13%) had disease that was neither high-risk nor progressive, 10 had disease that was either high-risk or refractory, and 11 had refractory, high-risk disease.
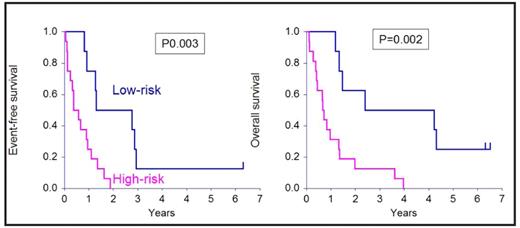
Four patients died of toxicity (3 GVHD, 1 multiorgan failure) and 19 relapsed. 18 of 19 relapsing patients died of progressive disease and 1 is alive in CR after a second allograft. High-risk (RR 0.11, P=0.0007) disease and refractoriness to therapy (RR=0.10, P=0.0006) were associated with poorer event-free survival. High-risk (RR 0.16, P=0.002) disease and refractoriness to therapy (RR=0.23, P=0.007) were associated with poorer event-free survival.
The combination of the two factors resulted in the identification of 3 categories of patients with highly disparate outcomes as shown in Figure 2.
shows the effect of the biological nature of the disease on EFS and OS.
Figure
Our data suggest that the outcome of allogeneic HSCT in myeloma is disappointing unless the disease is sensitive to therapy and biologically low-risk. However, such disease responds exceedingly well to autotransplantation–and is associated with prolonged survival that can rival that seen after allogeneic HSCT. Alternative strategies are needed to improve the outcome of patients with refractory or high-risk disease–and may include elective post-allograft maintenance therapy with novel agents such as thalidomide, lenalidomide or bortezomib.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal