Abstract
Background: Chronic red cell transfusions in patients with MDS result in iron overload, potentially causing hepatic, cardiac and endocrine dysfunction if untreated. DFX, a once-daily, oral iron chelator, has demonstrated dose-dependent reductions in SF and LIC in a variety of iron-overloaded patients. This study, CICL670AUS02, is the first prospective study evaluating the effects of DFX on LIC, SF and LPI in lower-risk patients with MDS.
Methods: The study evaluated efficacy and safety of DFX in patients with IPSS Low- and Int-1-risk MDS at three US centers. Initial DFX dose was 20 mg/kg/day with adjustments to 10 or 30 mg/kg/day based on safety and efficacy. LIC was assessed by R2 MRI at baseline (BL), 6 and 12 months (mos). SF, safety analyses and trough LPI levels were also assessed.
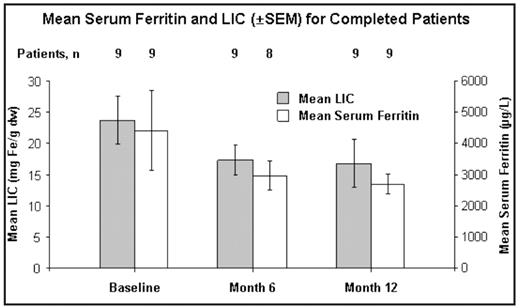
Baseline characteristics (n=24): Male/female ratio: 16/8; all Caucasian; mean age: 68 years (range 55–81); IPSS Low: 10; Int-1: 14. Time from initial diagnosis: <1 year (n=3; 13%); ≥1–<3 years (n=7; 29%); ≥3 years (n=14; 58%); range (<1–12) years. Mean number of lifetime transfusions was 79 (30–352). Mean (±SEM) SF value was 3848±583 μg/L, mean LIC was 20.64±1.99 mg Fe/g dry weight (dw), and mean LPI was 0.70±0.13 μmol/L; 50% of patients entered the trial with abnormal LPI (>0.5 μmol/L).
6-month values: 15/24 patients completed 6 mos. Mean SF (±SEM): 3650±384 μg/L at BL, 3638±870 μg/L at 6 mos (n=13); mean decrease of 12.2 μg/L (P=0.685). Mean LIC (±SEM): 23.10±2.40 mg Fe/g dw at BL, 16.87±1.88 mg Fe/g dw at 6 mos (n=14); mean decrease of 6.2 mg Fe/g dw (P=0.013). Mean LPI (±SEM): 0.82±0.16 μmol/L at BL, 0.12 ± 0.05 μmol/L at 6 mos (n=15); mean decrease of 0.70 μmol/L (P=0.170).
12-month values (n=9): Nine patients completed 12 mos of treatment. Mean dose in the nine completers was 20.1 mg/kg/day; three patients increased to 30 mg/kg/day and two decreased to 10 mg/kg/day. Mean SF (±SEM): 4416±1269 μg/L at BL, 2685±316 μg/L at 12 mos; mean decrease of 1730 μg/L (P=0.301; Figure). Mean LIC (±SEM): 23.72±3.82 mg Fe/g dw at BL, 16.79±3.80 mg Fe/g dw at 12 mos; mean decrease of 6.93 mg Fe/g dw (P=0.203). 78% of the nine completers achieved decreases in SF of ≥500 μg/L and >20% from BL. Mean LPI (±SEM; n=8): 0.82±0.19 μmol/L at BL, 0.13±0.10 μmol/L at 12 mos; mean decrease of 0.76 μmol/L (P=0.023)
Safety Analysis (n=24): Increases in serum creatinine (SCr) to >33% above BL on ≥2 occasions (highest value 1.7 mg/dL) were seen in seven patients (29%) while on study. New cases of thrombocytopenia (worst value 39×109/L) and neutropenia (worst value 0.50×109/L) developed in one patient each during the study; these were assessed as unrelated to study drug. There were 15 discontinuations (63%): three due to unrelated deaths (two congestive cardiac failure, one subdural hemorrhage); four patients withdrew consent for unknown reasons; two from MDS progression; five because of adverse events (AEs), four of which were related to study drug (one diarrhea, two increased SCr, one increased liver enzymes) and one unrelated (splenic infarct); and one from unsatisfactory therapeutic effect. There were 14 serious AEs reported in 11 patients; 13/14 were considered unrelated to study drug; 1/14 (severe diarrhea/lower abdominal cramping) was related to study drug and the patient completely recovered within 1 day.
Conclusions: Sequential LIC measurements in this MDS cohort demonstrated a significant decrease by month 6 and further decreases by month 12 in patients who completed 1 year of treatment. LPI normalized in 80% of the patients. Two thirds of this heavily transfused population discontinued the trial mainly due to disease-related complications; four patients discontinued due to drug-related AEs. Larger studies are continuing to evaluate the clinical benefit of DFX in patients with MDS.
Disclosures: Greenberg:Novartis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Amgen, GeminX: Research Funding. Schiffer:Novartis: Consultancy, Research Funding, Speakers Bureau. Koller:Novartis: Research Funding. Glynos:Novartis: Employment. Paley:Novartis: Employment.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal