Abstract
In vivo thrombosis imaging systems have been developed that show localized smallscale and short-acting thrombotic events in arterioles and venules; however, these microvascular models may not accurately simulate thrombogenesis in large vessels. A new quantitative imaging system was developed for evaluation of electrolytic-injury models of thrombus induction in large vessels of mice (carotid arteries and femoral veins). Laser-induced fluorophore emissions of membrane-labeled platelets and labeled fibrin-specific monoclonal antibodies were captured with time-lapse video recordings. Platelets were seen to accrue slowly after thrombus induction in both arteries and veins, peaking at approximately 15–45 minutes. In arteries, platelets accumulated more rapidly and to a greater extent than fibrin. Surprisingly, platelet accumulation in veins was substantial, occurring diffusely throughout the thrombus. Arterial thrombus dissolution occurred most often via large embolic events, sometimes occurring cyclically every 5–20 minutes by regrowth and re-embolization. In contrast, the rate of thrombus dissolution in veins was more gradual and appeared as micro-embolic events. Standard heparin anticoagulation inhibited venous thrombus growth by reducing fibrin development by 75% without affecting platelet accumulation. In contrast, blocking platelet aggregation at the αIIb/β3 receptor (with RGD-based mimetics) resulted in a 43% reduction in platelets and a surprising 5-fold increase in fibrin accumulation, suggesting the possibility of temporal competition between platelets and fibrin under venous conditions of thrombus formation. In established thrombi, newly activated platelets, identified by exposure of the active site of the αIIb/β3 receptor (using JON/A, a fluorophore-labeled antibody specific to activated αIIb/β3), were seen to roll in small aggregates across the outer margin of the thrombus mass in both arteries and veins for over 30 minutes, indicating continual dynamic platelet aggregation in both vessel types. Fibrin accrual was most intense at the margins of acute thrombotic stimuli and co-localized with fluorophore-labeled anti-Factor Xa and with Factor Va/VIIIa (identified using labeled activated protein C), which suggests that fibrin polymerization predominates adjacent to sites of these assembled coagulation complexes. In summary, the time-lapse nature of this video imaging system provides a unique and quantifiable method for determining how coagulation and platelet aggregation develop and are modulated in vivo within the same thrombus. Its use for the study of large vessel thrombosis often corroborated long-held tenets of platelet activity and coagulation, while revealing several novel findings:
platelets are major contributors to venous thrombosis;
arteries and veins have profound differences in thrombus dissolution/embolization patterns;
inhibiting platelet aggregation may augment fibrin clot formation;
platelet aggregation is a dynamic process in both arteries and veins, continually in flux on the thrombus surface for at least 30 minutes; and
fibrin forms local to sites of coagulation complex assembly.
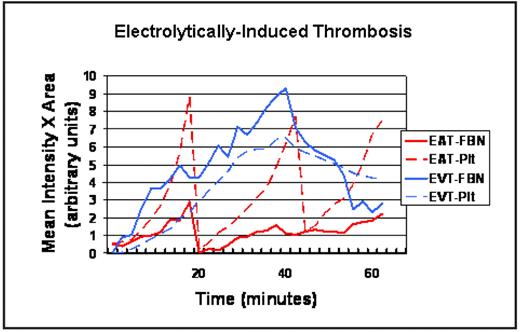
Graph illustrating single traces for representative electrolytically-induced, free-radical-mediated thrombi in mouse carotid arteries (EAT) and femoral veins (EVT) over 60 minutes, for anti-fibrin (FBN) and platelet (Plt) labels. The y-axis displays arbitrary units for the product of total thrombus area and average fluorophore intensity within the thrombus.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal