Abstract
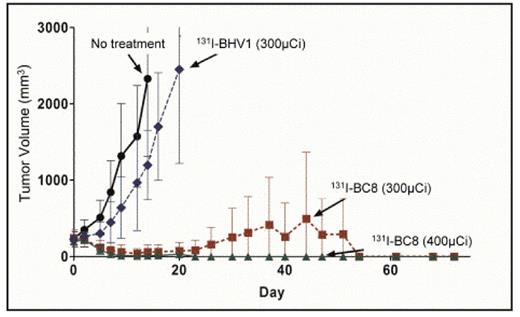
T-cell non-Hodgkin lymphoma (T-NHL) exhibits inferior remission durations and survival rates compared to B-cell NHL. This may be at least partially due to the paucity of targeted therapies and the absence of approved radioimmunoconjugates available for T-NHL. The CD45 antigen is expressed on ~90% of T-NHL and is not readily internalized or shed, making it an appealing target for radioimmunotherapy (RIT). In order to test this hypothesis, we first demonstrated that CD45 was expressed in high copy numbers on the surface of human T-NHL samples and T-NHL cell lines with a median of 3.4×105 CD45 molecules/cell on T-NHL lines (CCRF-CEM, Karpas 299) and 2.25×105 CD45 molecules/cell on patient derived T-NHL specimens (T-LBL, CTCL, NK/T-NHL). We employed an athymic murine xenograft model with the above human T-NHL lines to show that targeting human CD45 could deliver more radioactivity to tumor nodules than to normal tissues. Mice with palpable human T-NHL xenografts were injected with 131I-labeled BC8 (a murine anti-human CD45 antibody [Ab]) and an 125I-labeled murine isotype matched nonbonding control Ab (BHV1). CCRF-CEM xenografts targeted with 131I-BC8 demonstrated 86% (p=.003) and 106% (p=.001) more radioiodine retention at 24h and 48h, respectively, compared to xenografts targeted with the control BHV1 Ab. More importantly, tumor sites exhibited 1.6 (p=.045), 2.4 (p=.008), and 2.7 (p=.007)-fold 131I retention compared to the lungs, liver, and kidneys, respectively (CCRF-CEM, 24h). Similar results were observed at later time points and with other T-NHL lines. We next hypothesized that this preferential tumor targeting would translate into improved tumor control and survival using therapeutic doses of 131I-BC8. Mice with palpable T-NHL xenografts (CCRF-CEM) were randomly assigned to receive 200μg of BC8 labeled with either 300 or 400μCi of 131I, 200μg of BHV-1 (control) labeled with 300μCi 131I, or no treatment. Tumor dimensions and survival were tracked. By day 9, complete remissions (CR) were attained in 90% of mice treated with 400 μCi of 131I-BC8 and 67% of mice that received 300 μCi of 131I-BC8 (Figure). In contrast, none of the untreated control mice or mice that received 300 μCi of 131I-BHV-1 achieved CR (p<0.0001). All 20 mice in the 2 control groups required euthanasia due to tumor growth, whereas only 4 of 20 of the mice in experimental arms needed to be sacrificed due to disease progression and unmaintained remissions persisted for over 72 days following therapy. Since CD45 is also expressed on most normal hematopoietic tissues, we evaluated the ability of anti-CD45 RIT to preferentially localize to tumor and hematolymphoid sites in a syngeneic model targeting murine CD45. Mice with palpable EL-4 (murine T-NHL) xenografts were treated with 200 μg of 131I-30F11 (anti-murine CD45) followed by harvest of tumor sites and normal organs. In this syngeneic model, the ratios of retained radioactivity in tumor sites to critical non-target sites were: lung 5.3 (p<.001), liver 5.4 (p<.001), and kidney 8.7 (p<.001). Comparable ratios were seen when other target sites (marrow, spleen) were compared to normal non-target organs. These data indicate that CD45 is highly expressed on T-NHL, allows reliable antibody targeting of tumor sites in both xenogeneic and syngeneic models, and translates into significantly improved control of T-NHL xenografts.
These results support the hypothesis that CD45-targed RIT transplant conditioning regimens may improve outcomes for patients with relapsed/refractory T-NHL.
Tumor volumes of T-NHL xenografts after treatment with 131I-BC8 (anti-CD45), 131I-BHV1 (control), or no-treatment.
Tumor volumes of T-NHL xenografts after treatment with 131I-BC8 (anti-CD45), 131I-BHV1 (control), or no-treatment.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal