Abstract
Background: Multiple myeloma (MM) is a heterogeneous disease with very divergent outcomes that are dictated in a large part by specific cytogenetic abnormalities, as well as other prognostic factors such as the proliferative rate of marrow plasma cells. Prognostic systems incorporating these factors have shown clinical utility in identifying high-risk patients, and are increasingly being utilized for treatment decision-making. However, the prognostic relevance of these factors may change with the application of novel therapies. The objective of this study was to determine the impact of risk-stratification (incorporating plasma cell metaphase cytogenetics, interphase fluorescent in-situ hybridization (FISH) and the slide-based plasma cell labeling index (PCLI)) in a cohort of patients with newly diagnosed MM treated initially with lenalidomide + dexamethasone (Rev-Dex).
Methods: From March 2004 to November 2007, 100 consecutive patients treated with Rev (25mg/day) on days 1 through 21 of a 4-week cycle in combination with dexamethasone as initial therapy for newly diagnosed myeloma, were identified. High-risk MM was defined as presence of any one or more of the following: hypodiploidy, monoallelic loss of chromosome 13 or its long arm (by metaphase cytogenetics only), deletion of p53 (locus 17p13) or PCLI ≥ 3% or immunoglobulin heavy chain (IgH) translocations, t(4;14) (p16.3;q32) or t(14;16)(q32;q23) on FISH. PFS and OS survival estimates were created using the Kaplan Meier method, and compared by log-rank tests.
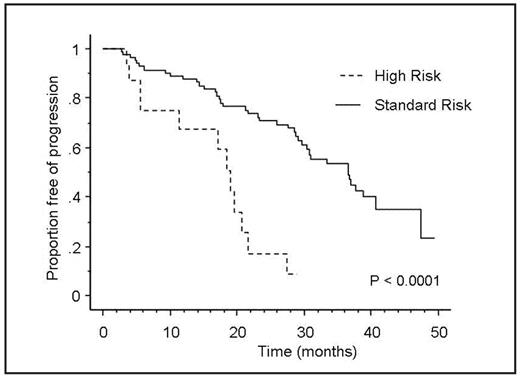
Results: The median estimated follow-up of the entire cohort (N=100) was 36 months. The median PFS was 31 months; the median OS has not been reached. The 2- and 3-year OS estimates were 93% and 83%, respectively. 16% patients were deemed high-risk by at least one of the 3 tests (cytogenetics, FISH or PCLI). Response rates (PR or better) were 81% versus 89% in the high-risk and standard risk groups, respectively, P=NS; corresponding values for CR plus VGPR rates were 38% and 45% respectively. The median PFS was 18.5 months in high-risk patients compared to 37 months in the standard-risk patients (n=84), P<0.001(Figure). Corresponding values for TTP were 18.5 months and 36.5 months, respectively, P=<0.001. OS was not statistically significant between the two groups; 92% 2-year OS was noted in both the groups. Overall, 95 patients had at least one of the 3 tests to determine risk, while 55 patients could be adequately stratified based on the availability of all the 3 tests, or at least one test result that led to their inclusion in the high-risk category. The significant difference in PFS persisted even when the analysis was restricted to the 55 patients classified using this stringent criterion; 18.5 months vs. 36.5 months in the high-risk and standard- risk groups respectively; P<0.001. In a separate analysis, patients who underwent SCT before the disease progression were censored on the date of SCT to negate its effect, and PFS was still inferior in the high-risk group (p=0.002).
Conclusion: The TTP and PFS of high-risk MM patients are inferior to that of the standard-risk patients treated with Rev-Dex, indicating that the current genetic and proliferation-based risk-stratification model remains prognostic with novel therapy. However, the TTP, PFS, and OS obtained in high-risk patients treated with Rev-Dex in this study is comparable to overall results in all myeloma patients reported in recent phase III trials. In addition, no significant impact of high-risk features on OS is apparent so far. Longer follow-up is needed to determine the impact of risk stratification on the OS of patients treated with Rev-Dex.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal