Abstract
We demonstrate that lck promoter–driven conditional expression of transgenic SPA-1, a Rap GTPase-activation protein, causes a profound defect of αβ T-cell development at the CD4/CD8 double-negative (DN) stage due to enhanced cell death without affecting γδ T-cell development. The effect was specific to the DN stage, because CD4 promoter–driven SPA-1 expression hardly affected T-cell development. Rap1A17, a dominant-negative Rap mutant, interfered with the generation of double-positive (DP) cells from Rag2−/− fetal thymocytes in vitro in the presence of anti-CD3ϵ antibody and Notch ligand. Rap GTPases were activated in a DN cell line by the expression of self-oligomerizing CD3 (CD8:CD3ϵ chimera), which substituted autonomous pre–T-cell receptor (TCR) signal, inducing CD69 expression and CD25 down-regulation. Reciprocally, expression of C3G, a Rap guanine nucleotide exchange factor, in both normal and Rag2−/− DN cells markedly enhanced Notch-dependent generation and expansion of DP cells without additional anti-CD3ϵ antibody, thus bypassing pre-TCR. Defective αβ T-cell development in the conditional SPA-1–transgenic mice was restored completely by introducing a p53−/− mutation. These results suggest that endogenous Rap GTPases downstream of pre-TCR play an essential role in rescuing pre-T cells from the p53-mediated checkpoint response, thus allowing Notch-mediated expansion and differentiation.
Introduction
T-cell development follows a precise sequence of events including several checkpoints. β-selection, the first checkpoint, occurs at the CD4/CD8 double-negative (DN) stage, in which thymocytes expressing pre-TCR consisting of pTα and T-cell receptor β (TCR-β) chains are rescued from cell death, followed by robust proliferation and differentiation to double-positive (DP) cells.1 The process selects and expands pre-T cells with successful recombination and expression of Tcrb, thus ensuring generation of a large repertoire of αβ TCRs. β-selection is crucially dependent on pre-TCR and Notch signaling.2-4 Pre-TCR signaling is delivered autonomously upon the proper assembly of pTα and TCRβ chains in a ligand-independent way and thus functions as a sensor of TCR-β chain expression.5,6 This feature has been attributed to the structural properties of pTα7,8 as well as the intrinsic hypersensitivity of pre-T cells to the receptor signal.9
Various types of small GTPases have been shown to play important roles at distinct stages of T-cell development including β-selection.10 It was reported that forced expression of oncogenic Ras mutant (RasV12) could induce the generation and expansion of DP cells from Rag1−/− DN cells.2,11 However, it was also indicated that DP cell development was hardly affected in lck-driven transgenic (Tg) mice of dominant-negative Ras (RasN17), whereas positive selection was severely compromised12 ; this result argues against an essential role of endogenous Ras signaling in β-selection. More recently it was reported that β-selection was affected partially and positive selection was impaired almost completely in ERK1,2−/− mice.13 Deficiency of Vav1, a guanine nucleotide exchange factor (GEF) for Rho family GTPases,14 caused a mild effect on β-selection, probably because of reduced pre-TCR signaling,15 and a more recent study revealed that mice deficient for all Vav family genes (Vav1, 2, and 3) showed a more profound defect in β-selection.16 On the other hand, it was reported that Tg expression of an active mutant of Rac1 could promote the transition from DN3 to DN4 stages of T-cell development.17,18 Most recently it was reported that mice deficient for both Rac1 and Rac2 showed a profoundly impaired development of DP cells.19 β-selection is a highly complex process consisting of multiple cellular events,1 and these results suggest that multiple small GTPases play crucial roles at distinct steps of β-selection.
Rap GTPases belong to the Ras family, which consist of Rap1 and Rap2 subfamilies20 that play important roles in T-cell activation and trafficking.21,22 It was reported previously that Tg expression of an active mutant of Rap1 (Rap1V12) hardly affected T-cell development per se23 ; however, involvement of endogenous Rap in T-cell development has not been investigated directly. We recently reported that the transplantation of hematopoietic progenitor cells expressing farnesylated C3G (C3G-F), a GEF capable of activating Rap1 and Rap2, caused markedly enhanced expansion of thymic DP cells; this was followed by frequent development of Notch-dependent T-cell leukemia.24 Here we report that the conditional expression of SPA-1 encoding a Rap-specific GTPase-activating protein (GAP) results in a profound and selective defect in β-selection without affecting positive selection of αβ T-lineage cells or γδ T-cell development. We demonstrate that Rap activation downstream of pre-TCR plays an indispensable role in rescuing DN cells from p53-dependent cell death, thus mediating a checkpoint response for TCRβ chain expression in pre-T cells.
Methods
Mice
The SPA-1 transgene was produced by ligating 3.2-kbp FLAG-tagged mouse SPA-1 cDNA into the SwaI cloning site of the pCALBIG vector, which was created by ligating an IRES-EGFP fragment into the EcoRI/ClaI sites of the pCALBL5 vector, a gift from Dr D. Kitamura (Tokyo University of Science, Chiba, Japan). A purified BamHI fragment of pCALBIG-FLAG-SPA-1 was injected into C57BL/6 (B6) mouse zygote pronuclei to generate SPA-1flox Tg mice. Two independent Tg founders (B4, C2) were used in the current study. Lck-Cre and CD4-Cre Tg mice were provided by Dr J. Takeda (Osaka University, Osaka, Japan) and Dr C. B. Wilson (University of Washington, Seattle), respectively. B6 mice were purchased from SLC (Shizuoka, Japan); Rag2−/− mice from Central Institute for Experimental Animals (Kanagawa, Japan); and p53−/− mice from Taconic Farm (Hudson, NY). SPA-1−/− mice were reported previously.25 All mice were maintained under pathogen-free conditions at the Institute of Laboratory Animals, Graduate School of Medicine, Kyoto University. All animal procedures described in this study were performed according to the guidelines for animal experiments of Kyoto University.
Cells and culture
The SCID.adh cell line, kindly provided by Dr D. L. Wiest (Fox Chase Cancer Center, Philadelphia, PA), and the TSt-4 and TSt-4/DLL1 stroma cell lines, provided by Dr H. Kawamoto (RIKEN Research Center for Allergy and Immunology, Yokohama, Japan), were maintained with RPMI 1640 supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, 100 μM nonessential amino acids, 1 mM sodium pyruvate, and antibiotics. The PLAT-E cell line, a kind gift from Dr T. Kitamura (University of Tokyo, Tokyo, Japan), was maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and antibiotics.
Antibodies
Monoclonal antibodies to CD3ϵ, CD4, CD8, CD25, CD44, CD69, TCRβ, TCRδ, human nerve growth factor receptor (NGFR), and annexin V were purchased from BD Biosciences (San Jose, CA). Anti-Rap1 and anti-Rap2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphorylated ERK and ERK antibodies were purchased from BD Biosciences. Anti-FLAG antibody was purchased from Sigma. Anti-phosphorylated AKT and anti-AKT antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Immunoblotting, immunoprecipitation, and pull-down assay
Cells were lysed with ice-cold lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.6], 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 10 mM NaF, 2 μg/mL leupeptin, 2 μg/mL aprotinin). Immunoblotting and immunoprecipitation were performed as described previously.26 RapGTP was detected by pull-down assay with the use of a GST fusion protein of the Rap-binding domain of RalGDS, followed by immunoblotting with an anti-Rap1 or anti-Rap2 antibody as described previously.26
Polymerase chain reaction
Cre-mediated recombination of the SPA-1 transgene was detected by genomic polymerase chain reaction (PCR; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) with the following primers: CAG-sense, gaccggcggctctagagcct; FLAG-antisense, tttgtcatcgtcgtccttgtagtc. Genomic TCR Dβ/Jβ rearrangements were detected with the following primers: TCRβ D2, gcacctgtggggaagaaact; TCRβ J2.6, tgagagctgtctcctactatcgatt. Total RNAs extracted using ISOGEN (NIPPON GENE) were treated with DNaseI (Invitrogen), and cDNAs were synthesized using Superscript III (Invitrogen). RT-PCR was performed with the following primers: pTα-sense, ctgcaactgggtcatgcttc; pTα-antisense, tcagaggggtgggtaagatc; gapdh-sense, gaacggatttggccgtattg; gapdh-antisense, gatgatgacccttttggctc.
Retrovirus production and infection
cDNA of SPA-1, Rap1A17, Rap1E63, RasN17, C3G-F (provided by Dr M. Matsuda, Kyoto University, Kyoto, Japan), or CD8:CD3ϵ chimera8 was subcloned into pMCs-ires-EGFP (MIG) or pMSCV-ires-human NGFR (MIN) retroviral vector. SPA-1 and Rap1E63 were FLAG-tagged, whereas Rap1A17 was untagged, since the tagging compromised the dominant-negative effect. PLAT-E packaging cells were transfected with the retrovirus plasmids, and the culture supernatants were harvested for the recombinant retrovirus. Cells were infected with the supernatants at 800g centrifugation in the presence of 4 μg/mL polybrene at 32°C for 60 minutes. Infected cells were identified with the use of green fluorescence protein (GFP) for MIG or anti-NGFR for MIN by FACSCalibur (BD Biosciences) and sorted by FACSVantage (BD Biosciences), if necessary. For double infection, SCID.adh cells were first infected with empty MIN or MIN containing SPA-1, Rap1N17, Rap1E63, or C3G-F, then NGFR+ cells were sorted and infected with empty MIG or MIG containing CD8:CD3ϵ.
In vitro thymocyte differentiation
Fetal thymocytes (embryonic day [E] 15–17) from B6, Rag2−/−, or SPA-1−/− mice were infected with empty MIG or MIG containing SPA-1, Rap1A17, RasN17, or C3G-F in the presence of 5 ng/mL interluekin-7 and Flk-L (Peprotech) for 1 hour. The cells were then cultured at 5 × 105 cells/well on semiconfluent TSt4 or TSt4/DLL1 cells. Anti-CD3ϵ mAb (10 μg/mL) was added 24 hours later, when indicated. In some experiments, the γ-secretase inhibitor, N-[N-(3,5-difluorophenacetyl-l-alanyl)]-(S)-phenyl-glycine t-butyl ester (DAPT; Calbiochem, San Diego, CA), was included in the culture at 1 μg/mL. At various days after the culture, the surface expression of CD4 and CD8 was analyzed by FACSCalibur.
Statistical analysis
Statistical analysis was performed using the 2-tailed Student t test.
Results
Expression of SPA-1 transgene selectively impairs thymic αβ T-cell development at the DN stage
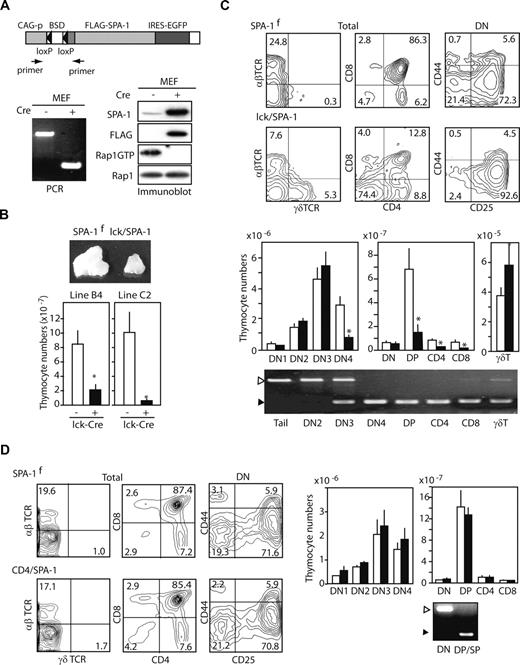
We previously reported that SPA-1−/− mice spontaneously develop a variety of myeloproliferative disorders25 as well as B-lineage leukemia in association with lupus-like autoimmune disease27 ; however, we found no abnormality in the thymic T-cell development.28 Whereas SPA-1 is a principal RapGAP in the hematopoietic progenitors,25 the thymocytes expressed several species of RapGAPs including SPA-1 and SPA-Ls (SPA-1–like genes; Figure S1). Thus, it was most probable that the possible role of endogenous Rap signal in T-cell development, if any, was overlooked in SPA-1−/− mice due to functional redundancy. In the current study, to investigate the role of endogenous Rap signal in T-cell development, we generated Tg mice using a cassette that encoded FLAG-tagged SPA-1 downstream of a floxed sequence (blasticidin S deaminase), which prevented transcription of SPA-1 via CAG promoter (SPA-1flox Tg mice; Figure 1A). Using the embryonic fibroblasts (MEFs) from SPA-1flox Tg mice, we confirmed that the introduction of Cre-recombinase resulted in the expression of SPA-1 transgene and the abrogation of endogenous RapGTP (Figure 1A). We then mated 2 independent SPA-1flox Tg lines (B4, C2) with lck-Cre Tg mice to generate double Tg (lck/SPA-1) mice. Both lck/SPA-1 Tg lines showed hypoplastic thymi with the mean thymocyte numbers being 25% and 5%, respectively, of those of control littermates (Figure 1B). The C2 line was used for later analysis. The proportion of DP cells was markedly reduced, and more than 90% of DN cells were arrested at the DN3 stage (Figure 1C). A majority of CD4 or CD8 single-positive (SP) population showed marginal CD3/TCR expression (data not shown), suggesting that they represented immature SP (ISP) cells. Absolute numbers of DN4, DP, and SP cells, in which Cre-mediated recombination was almost complete, declined significantly, whereas the number of DN3 cells was not affected, although they showed partial yet significant recombination. By contrast, the proportion and number of γδ T cells were rather increased (Figure 1C). To investigate whether or not the effect was specific to the DN stage of αβ T-cell development, we also generated CD4/SPA-1 Tg mice by crossing SPA-1flox Tg with CD4-Cre Tg mice. The numbers of total thymocytes (1.98 ± 0.10 × 108 vs 1.64 ± 0.11 × 108) as well as the proportions of thymocyte subsets were indistinguishable from those of control littermates, although the majority of DP and SP cells of CD4/SPA-1 Tg mice showed Cre-mediated recombination (Figure 1D). The results strongly suggest that, among the thymocytes, DN cells are selectively vulnerable to the transgenic expression of SPA-1.
Expression of SPA-1 transgene impairs the development of αβ T-lineage cells specifically at the DN3 to DN4 stage transition. (A) Schematic representation of the Tg vector (top). Primer positions to detect Cre-mediated recombination are indicated. MEFs from SPA-1flox Tg mice were infected with empty or Cre-containing adenovirus and then cultured for 2 days. DNA was extracted for the genomic PCR with the use of above primers (bottom left). Aliquots of the cells were lysed and subjected to immunoblotting with the indicated antibodies (bottom right). Rap1GTP was assessed by pull-down assay. (B) Two independent SPA-1flox Tg mouse lines, B4 and C2, were crossed with lck-Cre Tg mice, and the thymi of double Tg (lck/SPA-1) and littermate control mice were examined at 8 to 12 weeks of age. Representative macroscopic images and the mean thymocyte numbers of 5 mice are shown. *P < .01. (C) Representative FACS profiles of the thymocytes from lck/SPA-1 Tg and control littermates (top). The mean cell numbers and SE of the thymocyte subsets from 5 lck/SPA-1 Tg (■) and 5 littermate SPA-1flox Tg (□) mice are indicated. *P < .01. Thymocytes of each subset were sorted, and genomic PCR was done. ▷ indicates a flox band and ▶ a Cre-recombined band. (D) SPA-1flox Tg mice (C2 Tg mouse line) were crossed with CD4-Cre Tg mice, and the thymi of double Tg (CD4/SPA-1) and littermate control mice were examined at 5 to 6 weeks of age. Representative FACS profiles (left) and the mean cell numbers and SE of the thymocyte subsets from 4 CD4/SPA-1 Tg (■) and 4 littermate SPA-1flox Tg (□) mice are indicated (right). Thymocytes of DN and DP/SP fractions were sorted, and genomic PCR was done.
Expression of SPA-1 transgene impairs the development of αβ T-lineage cells specifically at the DN3 to DN4 stage transition. (A) Schematic representation of the Tg vector (top). Primer positions to detect Cre-mediated recombination are indicated. MEFs from SPA-1flox Tg mice were infected with empty or Cre-containing adenovirus and then cultured for 2 days. DNA was extracted for the genomic PCR with the use of above primers (bottom left). Aliquots of the cells were lysed and subjected to immunoblotting with the indicated antibodies (bottom right). Rap1GTP was assessed by pull-down assay. (B) Two independent SPA-1flox Tg mouse lines, B4 and C2, were crossed with lck-Cre Tg mice, and the thymi of double Tg (lck/SPA-1) and littermate control mice were examined at 8 to 12 weeks of age. Representative macroscopic images and the mean thymocyte numbers of 5 mice are shown. *P < .01. (C) Representative FACS profiles of the thymocytes from lck/SPA-1 Tg and control littermates (top). The mean cell numbers and SE of the thymocyte subsets from 5 lck/SPA-1 Tg (■) and 5 littermate SPA-1flox Tg (□) mice are indicated. *P < .01. Thymocytes of each subset were sorted, and genomic PCR was done. ▷ indicates a flox band and ▶ a Cre-recombined band. (D) SPA-1flox Tg mice (C2 Tg mouse line) were crossed with CD4-Cre Tg mice, and the thymi of double Tg (CD4/SPA-1) and littermate control mice were examined at 5 to 6 weeks of age. Representative FACS profiles (left) and the mean cell numbers and SE of the thymocyte subsets from 4 CD4/SPA-1 Tg (■) and 4 littermate SPA-1flox Tg (□) mice are indicated (right). Thymocytes of DN and DP/SP fractions were sorted, and genomic PCR was done.
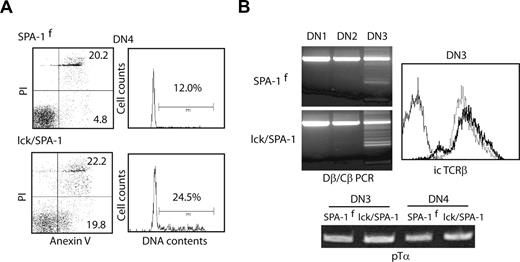
The DN4 cells of lck/SPA-1 Tg mice showed markedly increased apoptotic (PI−annexin V+) cells compared with those of control littermates, whereas they showed an even higher proportion of S and G2/M-phase cells (Figure 2A). The DN3 cells of lck/SPA-1 Tg mice exhibited Dβ/Jβ rearrangement and intracytoplasmic TCR-β chain expression comparable with the control littermates; the expression of pTα transcripts in DN3 and DN4 cells was also unaltered (Figure 2B). The results suggest that the defect of αβ T cell development is attributed to the enhanced cell death at the DN stage in spite of the expression of pre-TCR. Being consistent with DN cell death, expression of the Tg SPA-1 protein was barely detected by regular biochemical analysis (data not shown). Peripheral αβ T cells of lck/SPA-1 Tg mice were also reduced, and the residual splenic αβ T cells in the spleen revealed no detectable Cre-mediated recombination, thus representing “recombination-leaked” cells (Figure S2). On the contrary, the numbers of peripheral γδ T cells were increased more than 2-fold (Figure S2).
Enhanced apoptosis in situ of the DN4 cells from lck/SPA-1 Tg mice. (A) DN4 thymocytes isolated from lck/SPA-1 Tg and SPA-1flox Tg littermate mice were immediately 2-color stained with PI and annexin V (left), or fixed and stained for DNA with PI (right). (B) Subsets of DN thymocytes were sorted from lck/SPA-1 Tg and littermate control mice, and genomic PCR was done with the use of TCR Dβ and Jβ primers (top left). The DN3 cells were fixed, permealized and stained with anti-TCRβ antibody (top right). Solid line indicates lck/SPA-1Tg; dotted line, SPA-1flox Tg mice; and fine line, staining with control antibody. RNAs were extracted from DN3 and DN4 cells, and RT-PCR was done with the use of pTα primers (bottom).
Enhanced apoptosis in situ of the DN4 cells from lck/SPA-1 Tg mice. (A) DN4 thymocytes isolated from lck/SPA-1 Tg and SPA-1flox Tg littermate mice were immediately 2-color stained with PI and annexin V (left), or fixed and stained for DNA with PI (right). (B) Subsets of DN thymocytes were sorted from lck/SPA-1 Tg and littermate control mice, and genomic PCR was done with the use of TCR Dβ and Jβ primers (top left). The DN3 cells were fixed, permealized and stained with anti-TCRβ antibody (top right). Solid line indicates lck/SPA-1Tg; dotted line, SPA-1flox Tg mice; and fine line, staining with control antibody. RNAs were extracted from DN3 and DN4 cells, and RT-PCR was done with the use of pTα primers (bottom).
Crucial role of endogenous Rap signaling in β-selection
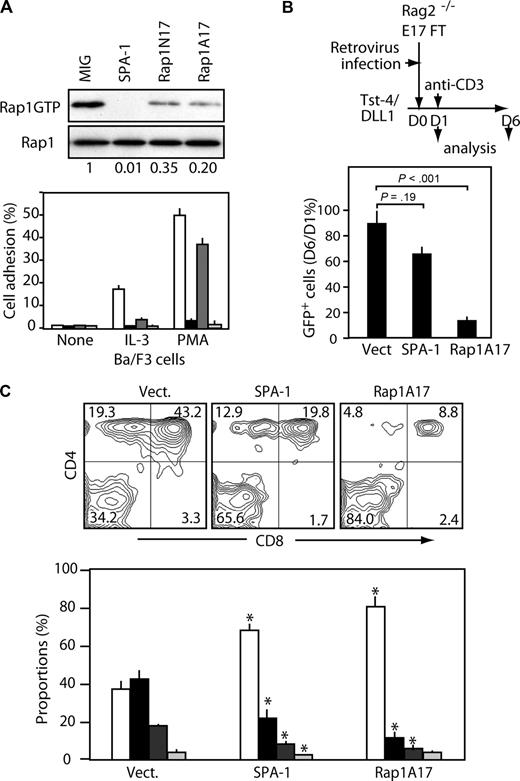
We then directly investigated the effects of a recently described dominant-negative Rap1 mutant (Rap1A17), which selectively interferes with the GEF activity of C3G and Epac,29 on early T-cell development with the use of the in vitro differentiation assay.2 Rap1A17 exhibited a more potent dominant negative effect than the widely used Rap1N17 in the cells (Figure 3A). Fetal thymocytes from Rag2−/− mice were infected with empty MIG or with MIG containing SPA-1 or Rap1A17, and then cultured in the presence of thymic stroma cells expressing Notch ligand delta-like 1 (TSt4/DLL1) together with anti-CD3ϵ antibody that mimicked pre-TCR signal.2 To make sure that the stimulation by anti-CD3ϵ antibody was initiated after the full retroviral gene expression, the antibody was added 1 day after the retrovirus infection (Figure 3B). The levels of GFP expression at day 1 varied from 10% to 25%. On day 6 of culture, the recovery of viable control GFP+ cells was comparable to that on day 1; however, as much as 60% of the cells were converted to DP/CD4 ISP phenotype (Figure 3B,C). On the other hand, the numbers of viable SPA-1/GFP+ and Rap1A17/GFP+ cells were markedly lower on day 6, and the residual cells showed markedly reduced conversion to DP/CD4 ISP cells, 30% and 14%, respectively, compared with the control cells (Figure 3B,C). In all groups, GFP− populations showed essentially identical profiles to that of the vector control (data not shown). We thus conclude that endogenous Rap activation plays a crucial role in the survival and DP conversion of DN cells.
Dominant-negative Rap (Rap1A17) inhibits β-selection of Rag2−/− DN cells in vitro. (A) Ba/F3 cell line was infected with empty MIG (□) or MIG containing SPA-1 ( ), Rap1N17 (
), Rap1N17 ( ), or Rap1A17 (■). The GFP+ cells were sorted, cultured in the presence of interleukin-3, and lysed. Rap1GTP was assessed by pull-down assay, and Rap1 activation indices are indicated (top). Aliquots of the cells were fluorescence-labeled and cultured in the absence or presence of interleukin-3 or PMA on fibronectin-coated plates for 30 minutes, then the fluorescence of cells adherent to fibronectin was examined (bottom). Means and SE of triplicate cultures are indicated. (B) Fetal thymocytes (FT) from Rag2−/− mice (E17) were infected with empty MIG or MIG containing SPA-1 or Rap1A17 and cultured on TSt4/DLL1 stroma cells (top). One day later, 10 μg/mL anti-CD3ϵ antibody was added. The cells were harvested on days 1 and 6. Mean viable cell recoveries and SE of GFP+ cells at day 6 versus day 1 in 5 independent experiments are indicated (bottom). (C) The cells at day 6 in panel B were 2-color stained with anti-CD4 and anti-CD8; the representative profiles in a GFP+ gate are shown (top). The mean proportions and SE of DN (□), DP (■), CD4 SP (
), or Rap1A17 (■). The GFP+ cells were sorted, cultured in the presence of interleukin-3, and lysed. Rap1GTP was assessed by pull-down assay, and Rap1 activation indices are indicated (top). Aliquots of the cells were fluorescence-labeled and cultured in the absence or presence of interleukin-3 or PMA on fibronectin-coated plates for 30 minutes, then the fluorescence of cells adherent to fibronectin was examined (bottom). Means and SE of triplicate cultures are indicated. (B) Fetal thymocytes (FT) from Rag2−/− mice (E17) were infected with empty MIG or MIG containing SPA-1 or Rap1A17 and cultured on TSt4/DLL1 stroma cells (top). One day later, 10 μg/mL anti-CD3ϵ antibody was added. The cells were harvested on days 1 and 6. Mean viable cell recoveries and SE of GFP+ cells at day 6 versus day 1 in 5 independent experiments are indicated (bottom). (C) The cells at day 6 in panel B were 2-color stained with anti-CD4 and anti-CD8; the representative profiles in a GFP+ gate are shown (top). The mean proportions and SE of DN (□), DP (■), CD4 SP ( ), and CD8 SP (
), and CD8 SP ( ) cells in 5 independent experiments are indicated (bottom). *P < .01 compared with the proportions of corresponding subsets in vector control group.
) cells in 5 independent experiments are indicated (bottom). *P < .01 compared with the proportions of corresponding subsets in vector control group.
Dominant-negative Rap (Rap1A17) inhibits β-selection of Rag2−/− DN cells in vitro. (A) Ba/F3 cell line was infected with empty MIG (□) or MIG containing SPA-1 ( ), Rap1N17 (
), Rap1N17 ( ), or Rap1A17 (■). The GFP+ cells were sorted, cultured in the presence of interleukin-3, and lysed. Rap1GTP was assessed by pull-down assay, and Rap1 activation indices are indicated (top). Aliquots of the cells were fluorescence-labeled and cultured in the absence or presence of interleukin-3 or PMA on fibronectin-coated plates for 30 minutes, then the fluorescence of cells adherent to fibronectin was examined (bottom). Means and SE of triplicate cultures are indicated. (B) Fetal thymocytes (FT) from Rag2−/− mice (E17) were infected with empty MIG or MIG containing SPA-1 or Rap1A17 and cultured on TSt4/DLL1 stroma cells (top). One day later, 10 μg/mL anti-CD3ϵ antibody was added. The cells were harvested on days 1 and 6. Mean viable cell recoveries and SE of GFP+ cells at day 6 versus day 1 in 5 independent experiments are indicated (bottom). (C) The cells at day 6 in panel B were 2-color stained with anti-CD4 and anti-CD8; the representative profiles in a GFP+ gate are shown (top). The mean proportions and SE of DN (□), DP (■), CD4 SP (
), or Rap1A17 (■). The GFP+ cells were sorted, cultured in the presence of interleukin-3, and lysed. Rap1GTP was assessed by pull-down assay, and Rap1 activation indices are indicated (top). Aliquots of the cells were fluorescence-labeled and cultured in the absence or presence of interleukin-3 or PMA on fibronectin-coated plates for 30 minutes, then the fluorescence of cells adherent to fibronectin was examined (bottom). Means and SE of triplicate cultures are indicated. (B) Fetal thymocytes (FT) from Rag2−/− mice (E17) were infected with empty MIG or MIG containing SPA-1 or Rap1A17 and cultured on TSt4/DLL1 stroma cells (top). One day later, 10 μg/mL anti-CD3ϵ antibody was added. The cells were harvested on days 1 and 6. Mean viable cell recoveries and SE of GFP+ cells at day 6 versus day 1 in 5 independent experiments are indicated (bottom). (C) The cells at day 6 in panel B were 2-color stained with anti-CD4 and anti-CD8; the representative profiles in a GFP+ gate are shown (top). The mean proportions and SE of DN (□), DP (■), CD4 SP ( ), and CD8 SP (
), and CD8 SP ( ) cells in 5 independent experiments are indicated (bottom). *P < .01 compared with the proportions of corresponding subsets in vector control group.
) cells in 5 independent experiments are indicated (bottom). *P < .01 compared with the proportions of corresponding subsets in vector control group.
Activation of Rap GTPases via pre-TCR
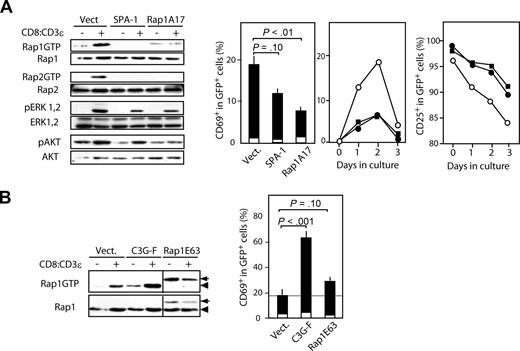
We next investigated whether or not Rap was activated directly via pre-TCR signal. To this end, we used self-oligomerizing CD3ϵ (chimeric CD8:CD3ϵ), which could substitute autonomous pre-TCR signaling in DN cells.8 We first transfected a DN cell line (SCID.adh) with SPA-1 or Rap1A17 by means of NGFR-containing retroviral vector (MIN); the sorted NGFR+ cells were subsequently infected with empty MIG or MIG containing CD8:CD3ϵ. Expression of CD8:CD3ϵ induced activation of both Rap1 and Rap2 in control cells, whereas those expressing SPA-1 or Rap1A17 showed markedly reduced Rap activation after CD8:CD3ϵ expression (Figure 4A). ERK as well as AKT activation was also compromised in SCID.adh cells expressing SPA-1 or Rap1A17 compared with control cells (Figure 4A). Concomitantly, the induction of CD69 expression as well as the reduction of CD25 expression after CD8:CD3ϵ expression was significantly compromised in the cells expressing SPA-1 or Rap1A17; this result was not ascribed to the altered activation kinetics (Figure 4A). These results strongly suggest that Rap activation is induced downstream of pre-TCR and mediates the effects at least in part in a DN cell line. In reciprocal experiments, we transfected SCID.adh cells with C3G-F or an active mutant of Rap1 (Rap1E63). SCID.adh cells expressing C3G-F showed enhanced Rap1 activation after CD8:CD3ϵ expression; this result was paralleled by markedly augmented CD69 induction (Figure 4B). On the other hand, SCID.adh cells expressing Rap1E63 showed augmented amounts of basal Rap1GTP, with CD8:CD3ϵ expression resulting in little additional increase (Figure 4B). Although these cells also showed enhanced CD69 expression, the extent was less than that of cells expressing C3G-F (Figure 4B). Because C3G-F is efficiently targeted to the plasma membrane compared with Rap1E63,26,30 it may be suggested that Rap activation at the plasma membrane is important for pre-TCR signaling.
Rap activation mediates the pre-TCR signal in a DN cell line. (A) SCID.adh cells were infected with empty MIN or MIN containing SPA-1 or Rap1N17, and NGFR+ cells were sorted. The cells were then infected with empty MIG or MIG containing CD8:CD3ϵ and cultured. Sixteen hours later the cells were harvested, lysed, and immunoblotted with the indicated antibodies (left). RapGTPs were detected by a pull-down assay. Similar results were obtained in 2 independent analyses. Twenty-eight hours after the culture, the cells were harvested and stained with anti-CD69 antibody. The means and SE of the proportions of CD69+ cells in a GFP+ gate of 5 independent experiments are indicated (middle). □, empty MIN; ■, MIN-CD8:CD3ϵ. To follow the kinetics, the cells were harvested at various days after CD8:CD3ϵ expression and the proportions of CD69+ and CD25+ cells in GFP+ gate were determined (right), SCID.adh cells transfected with empty MIN (○), SPA-1 (●), and Rap1A17 (■). Similar results were obtained in 2 independent experiments. (B) SCID.adh cells were infected with empty MIN or MIN containing C3G-F or FLAG-tagged Rap1E63, and the similar analysis described in panel A were performed.  indicates FLAG-tagged Rap1E63; and ◀, endogenous Rap1.
indicates FLAG-tagged Rap1E63; and ◀, endogenous Rap1.
Rap activation mediates the pre-TCR signal in a DN cell line. (A) SCID.adh cells were infected with empty MIN or MIN containing SPA-1 or Rap1N17, and NGFR+ cells were sorted. The cells were then infected with empty MIG or MIG containing CD8:CD3ϵ and cultured. Sixteen hours later the cells were harvested, lysed, and immunoblotted with the indicated antibodies (left). RapGTPs were detected by a pull-down assay. Similar results were obtained in 2 independent analyses. Twenty-eight hours after the culture, the cells were harvested and stained with anti-CD69 antibody. The means and SE of the proportions of CD69+ cells in a GFP+ gate of 5 independent experiments are indicated (middle). □, empty MIN; ■, MIN-CD8:CD3ϵ. To follow the kinetics, the cells were harvested at various days after CD8:CD3ϵ expression and the proportions of CD69+ and CD25+ cells in GFP+ gate were determined (right), SCID.adh cells transfected with empty MIN (○), SPA-1 (●), and Rap1A17 (■). Similar results were obtained in 2 independent experiments. (B) SCID.adh cells were infected with empty MIN or MIN containing C3G-F or FLAG-tagged Rap1E63, and the similar analysis described in panel A were performed.  indicates FLAG-tagged Rap1E63; and ◀, endogenous Rap1.
indicates FLAG-tagged Rap1E63; and ◀, endogenous Rap1.
Forced activation of endogenous Rap enhances Notch-dependent β-selection bypassing pre-TCR
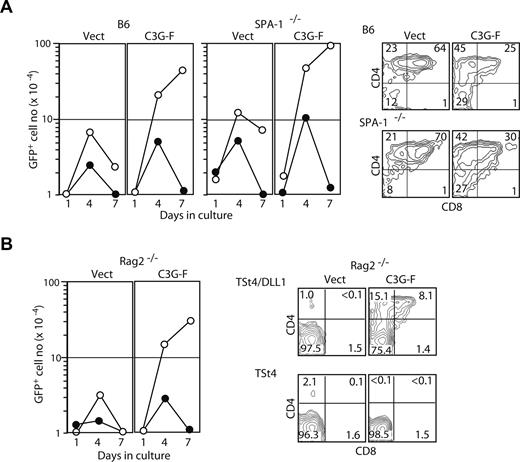
We then investigated the effect of forced activation of endogenous Rap in normal DN cells. To this end, DN cells from fetal thymocytes of B6 or SPA-1−/− mice were infected with empty MIG or MIG containing C3G-F, and then cultured in the presence of TSt4/DLL-1. C3G-F/GFP+ cells showed markedly enhanced and persistent proliferation until day 7, whereas the proliferation of control GFP+ cells was transient, peaking on day 4 (Figure 5A). Being consistent with the involvement of endogenous Rap activation, C3G-F/GFP +SPA-1−/− DN cells revealed an even more striking proliferation (Figure 5A). It was confirmed that the C3G-F/GFP+ DN cells from both groups showed significant DP conversion (Figure 5A). Although the proportions of DP cells in the C3G-F/GFP+ cells were less than those in control GFP+ cells on day 4, the absolute DP cell numbers generated in the cultures were much greater. The robust proliferation of both control GFP+ and C3G-F/GFP+ DN cells was completely inhibited in the presence of a γ-secretase inhibitor (DAPT) on day 7, suggesting that the proliferation and differentiation remained dependent on Notch-signaling (Figure 5A). To examine the requirement of pre-TCR, we performed the same experiments using Rag2−/− DN cells. Whereas control GFP+ Rag2−/− DN cells showed only marginal and transient proliferation in the absence of anti-CD3ϵ antibody with no generation of DP cells, C3G-F/GFP+ Rag2−/− DN cells revealed robust proliferation accompanied by significant DP conversion, although the DP cell proportion was less than that in B6 DN cells (Figure 5B). The proliferation was again inhibited in the presence of DAPT; also, C3G-F/GFP+ Rag2−/− DN cells showed no significant DP conversion and much less survival, when they were cultured with TSt4 cells without expressing DLL1 (1.5 ± 0.3 × 105 cells in the presence of TSt4 vs 8.5 ± 1.5 × 105 cells in the presence of TSt4-DLL1 on day 4; Figure 5B). The results suggest that forced activation of endogenous Rap in normal DN cells can bypass pre-TCR, but not Notch, signaling to induce proliferation and differentiation.
Expression of C3G-F enhances Notch-dependent β-selection in vitro bypassing pre-TCR. (A) Fetal thymocytes (E17) from B6 or SPA-1−/− mice were infected with empty MIG or MIG containing C3G-F and cultured on TSt4/DLL1 cells in the absence (○) or presence (●) of DAPT (1 μg/mL). The cells were harvested on day 1, day 4, and day 7, and the GFP+ cell numbers were determined (left). The experiments were repeated twice, and similar results were obtained. On day 4, aliquots of the cells were harvested and 2-color stained with anti-CD4 and anti-CD8 antibodies; the profiles in a GFP+ gate are indicated (right). (B) Fetal thymocytes (E17) from Rag2−/− mice were infected with empty MIG or MIG containing C3G-F, and then cultured on TSt4/DLL1 cells in the absence (○) or presence (●) of DAPT (1 μg/mL) without the addition of anti-CD3ϵ antibody. The cells were harvested on day 1, day 4, and day 7, and the GFP+ cell numbers were determined (left). Similar results were obtained in 2 independent experiments. The cells were cultured in the presence of TSt4 or TSt4/DLL1 cells and stained with anti-CD4 and anti-CD8 antibodies on day 4; the profiles in a GFP+ gate are indicated (right).
Expression of C3G-F enhances Notch-dependent β-selection in vitro bypassing pre-TCR. (A) Fetal thymocytes (E17) from B6 or SPA-1−/− mice were infected with empty MIG or MIG containing C3G-F and cultured on TSt4/DLL1 cells in the absence (○) or presence (●) of DAPT (1 μg/mL). The cells were harvested on day 1, day 4, and day 7, and the GFP+ cell numbers were determined (left). The experiments were repeated twice, and similar results were obtained. On day 4, aliquots of the cells were harvested and 2-color stained with anti-CD4 and anti-CD8 antibodies; the profiles in a GFP+ gate are indicated (right). (B) Fetal thymocytes (E17) from Rag2−/− mice were infected with empty MIG or MIG containing C3G-F, and then cultured on TSt4/DLL1 cells in the absence (○) or presence (●) of DAPT (1 μg/mL) without the addition of anti-CD3ϵ antibody. The cells were harvested on day 1, day 4, and day 7, and the GFP+ cell numbers were determined (left). Similar results were obtained in 2 independent experiments. The cells were cultured in the presence of TSt4 or TSt4/DLL1 cells and stained with anti-CD4 and anti-CD8 antibodies on day 4; the profiles in a GFP+ gate are indicated (right).
Rap signal rescues DN cells from p53-mediated cell death
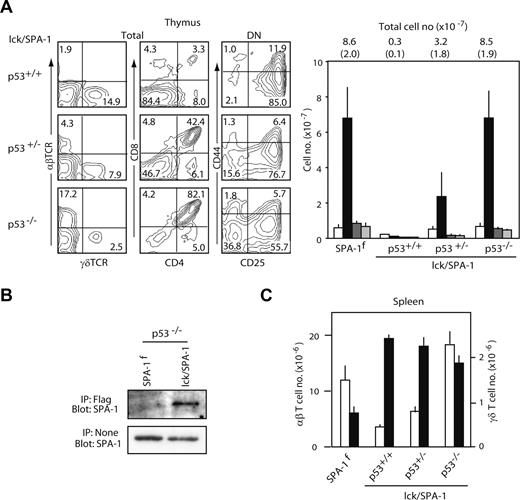
To investigate possible involvement of p53 in the defective β-selection of αβ T-lineage cells in lck/SPA-1 Tg mice, we crossed lck/SPA-1 Tg with p53−/− mice. Although p53+/+ lck/SPA-1 littermate mice showed a profound defect in DP cell development as expected, the thymi of p53−/− lck/SPA-1 mice revealed the total thymocyte numbers as well as the proportions of the subsets essentially comparable to those of SPA-1flox littermates (Figure 6A). p53+/− lck/SPA-1 mice showed partial restoration of DP cell development, indicating that the effect was dependent on the dosages of p53 gene (Figure 6A). FLAG-tagged SPA-1 protein could be clearly detected in the thymocytes from p53−/− lck/SPA-1 Tg mice; the result was consistent with the rescue of the thymocytes expressing the SPA-1 transgene from cell death (Figure 6B). The numbers of splenic αβ T cells were also restored in p53−/− and p53+/− lck/SPA-1 Tg mice completely and partially, respectively, and those of γδ T cells tended to decline, but not to the control level (Figure 6C): a slight increase in the numbers of both αβ and γδ T cells in the spleen of p53−/− lck/SPA-1 Tg mice compared with SPA-1flox littermates may reflect the effect of p53 deficiency at the periphery. We thus conclude that Rap signal is essential in rescuing DN cells from p53-dependent cell death in β-selection.
Introduction of p53−/− mutation restores the defect of αβ T-cell development in lck/SPA-1 Tg mice. (A) The thymocytes from lck/SPA-1 Tg mice of p53+/+, p53+/−, or p53−/− backgrounds at 5 weeks of age (4 mice per group) were stained with the combinations of indicated antibodies; the representative FACS profiles are indicated (left). The mean numbers and SE (parentheses) of the total thymocytes as well as the subsets are also indicated (bottom); DN (□), DP (■), CD4 SP ( ) and CD8 SP (
) and CD8 SP ( ) cells. (B) Thymocytes from p53−/− lck/SPA-1 Tg and control SPA-1flox mice were lysed, immunoprecipitated with anti-FLAG, and blotted with anti–SPA-1 antibody. (C) Spleen cells were 2-color stained with anti-TCRβ and anti-TCRδ antibodies, and the mean numbers and SE of αβ T cells (□) and γδ T cells (■) were determined.
) cells. (B) Thymocytes from p53−/− lck/SPA-1 Tg and control SPA-1flox mice were lysed, immunoprecipitated with anti-FLAG, and blotted with anti–SPA-1 antibody. (C) Spleen cells were 2-color stained with anti-TCRβ and anti-TCRδ antibodies, and the mean numbers and SE of αβ T cells (□) and γδ T cells (■) were determined.
Introduction of p53−/− mutation restores the defect of αβ T-cell development in lck/SPA-1 Tg mice. (A) The thymocytes from lck/SPA-1 Tg mice of p53+/+, p53+/−, or p53−/− backgrounds at 5 weeks of age (4 mice per group) were stained with the combinations of indicated antibodies; the representative FACS profiles are indicated (left). The mean numbers and SE (parentheses) of the total thymocytes as well as the subsets are also indicated (bottom); DN (□), DP (■), CD4 SP ( ) and CD8 SP (
) and CD8 SP ( ) cells. (B) Thymocytes from p53−/− lck/SPA-1 Tg and control SPA-1flox mice were lysed, immunoprecipitated with anti-FLAG, and blotted with anti–SPA-1 antibody. (C) Spleen cells were 2-color stained with anti-TCRβ and anti-TCRδ antibodies, and the mean numbers and SE of αβ T cells (□) and γδ T cells (■) were determined.
) cells. (B) Thymocytes from p53−/− lck/SPA-1 Tg and control SPA-1flox mice were lysed, immunoprecipitated with anti-FLAG, and blotted with anti–SPA-1 antibody. (C) Spleen cells were 2-color stained with anti-TCRβ and anti-TCRδ antibodies, and the mean numbers and SE of αβ T cells (□) and γδ T cells (■) were determined.
Discussion
In this study, we investigated the role of Rap GTPases in T-cell development. The lck/SPA-1 Tg mice showed markedly hypoplastic thymi, in which the development of αβ T-lineage cells was arrested at the transition from DN3 to DN4 stage with that of γδ T cells being unaffected. The DN4 cells revealed enhanced apoptosis in situ; accordingly mature αβ T cells showing Cre recombination and thus expressing SPA-1 transgene were rarely detected in the peripheral lymphoid tissues, whereas γδ T cells were rather increased. The results suggest the failure of β-selection process in lck/SPA-1 Tg mice. β-selection can be recapitulated in vitro by culturing Rag2−/− DN cells in the presence of stroma cells expressing Notch ligands together with anti-CD3ϵ antibody that mimicks pre-TCR signaling.2 With the use of the culture system, current results further demonstrate that retroviral expression of SPA-1 or a dominant-negative Rap mutant (Rap1A17) in Rag2−/− DN cells strongly inhibited the survival and proliferation as well as the conversion to DP cells. The results strongly suggest that the phenotypes of lck/SPA-1 Tg mice were indeed due to the inhibition of endogenous Rap signaling in DN cells. In strong contrast, CD4/SPA-1 Tg mice showed T-cell development indistinguishable from control mice; thus, Tg SPA-1 expression in DP cells hardly affected the positive or negative selection. Collectively, current results strongly suggest that endogenous Rap signaling plays an indispensable role specifically in β-selection of αβ T-lineage cell development. The role of Rap signaling appears quite distinct from that of endogenous Ras signaling in T-cell development, in that lck/RasN17 Tg mice were reported to show a severe defect in positive selection with little effect on β-selection.12
Pre-TCR autonomously signals in a ligand-independent way, and the signaling can be substituted by the expression of a self-dimerizing CD8:CD3ϵ chimeric protein in DN cells.8 Current results revealed that the expression of CD8:CD3ϵ activated both Rap1 and Rap2 in SCID.adh DN cells. SCID.adh cells expressing SPA-1 or Rap1A17, however, showed markedly impaired Rap activation after CD8:CD3ϵ expression; they also showed reduced ERK and AKT activation as well as compromised CD69 induction and CD25 down-regulation. The results strongly suggest an important role of Rap activation in the autonomous pre-TCR signaling. Reciprocally, expression of membrane-targeted C3G (C3G-F) strongly enhanced Rap activation and caused a marked augmentation of CD69 induction after CD8:CD3ϵ expression. Interestingly, the effect was by far more prominent than that by the expression of an active Rap mutant (Rap1E63). Whereas C3G-F is tethered efficiently to the plasma membrane and activated there via Crk in couple with receptors,26,30 Rap1E63 may contribute to the increase in basal Rap1GTP level diffusely in the cytosol.31 In this aspect, it is also noted that a significant proportion of SPA-1 is anchored to the plasma membrane.32 The results thus may support a view that endogenous Rap activation at the plasma membrane is important for pre-TCR signaling. Because Rap regulators, including SPA-1 and C3G as well as Rap1A17, affect the activation of both endogenous Rap1 and Rap2, it remains to be seen whether or not they function similarly downstream of pre-TCR.
Among complex events during β-selection, a crucial one is to rescue the DN cells that successfully express pre-TCR from programmed cell death.1 It was reported that impaired β-selection in mutant mice with defective pre-TCR expression, such as CD3γ−/−, Eβ−/−, and scid/scid mice, was restored by introducing p53−/− mutation.33-35 Prolonged TCR Vβ (Dβ)Jβ gene recombination events in scid and Eβ−/− mutations were reported to activate the p53-dependent checkpoint for DNA damage.34,35 These results led to the hypothesis that pre-TCR may deliver a signal to suppress emerging p53 activity,36 although the actual stresses inducing the p53-mediated checkpoint response in normal DN cells remain to be elucidated. Recent reports indicate that ribosomal proteins such as S6 and L22 are involved in controlling the p53-dependent checkpoint response in DN cells.37,38 Molecular links between the pre-TCR expression and p53-mediated checkpoint response in normal DN cells, however, remain unknown. Current results demonstrate that αβ T-cell development in lck/SPA-1 Tg mice is restored completely by introducing the p53−/− mutation, strongly suggesting that Rap signal plays an essential role in rescuing pre-T cells from p53-dependent cell death. So far, we have failed to detect the expression of p53 protein in normal DN cells by regular biochemical techniques, and possible effects of Rap signal on p53 protein levels remain to be further pursued. Alternatively, it may be possible that Rap signaling affects the apoptotic pathway downstream of activated p53. For instance, it was reported recently that AKT1,2−/− mice showed a profound defect in β-selection attributable to the death of DN cells,39 and the current results indicated that CD8:CD3ϵ expression induces AKT activation in a DN cell line, which is compromised by SPA-1 overexpression. Given that AKT activation inhibits p53-induced apoptosis,40 one is tempted to speculate that the Rap signal may counteract p53 activity in part via AKT activation.
Current results further suggest that Rap signal may show an additional effect on β-selection. Thus, expression of C3G-F in normal DN cells causes a marked enhancement of the proliferation and DP cell generation in a Notch-dependent manner in vitro; the effect is more striking in SPA-1−/− DN cells as expected. Importantly, significant Notch-dependent expansion and DP cell generation was seen also in C3G-F+ Rag2−/− DN cells even in the absence of anti-CD3ϵ antibody, thus bypassing pre-TCR signal. A similar synergistic effect on Notch-mediated differentiation of DN cells to DP cells has been reported by the exogenous expression of oncogenic RasV12 in vitro2 ; the efficiency appears even more striking than C3G-F expression. Thus, the synergistic effect on the Notch function may be redundant between Ras and Rap GTPases. Nonetheless, we recently reported that SPA-1−/− hematopoietic progenitors expressing C3G-F caused marked thymic hyperplasia and frequent development of Notch-dependent T-cell acute lymphoblastic leukemia (T-ALL) with activating notch mutations.24 The T-ALL arose exclusively in the thymus, and the majority of them showed DP phenotype. Thus, the synergistic effect of Rap on Notch signal in DN cells may be important with respect to T-ALL genesis.
It has been argued that, among thymocytes, DN cells may be intrinsically “hypersensitive” to receptor signaling compared with DP and SP cells.8,9 Intriguingly, Rap and Ras play indispensable roles in β-selection of DN cells and positive selection of DP cells, respectively, during αβ T-cell development; thus, the apparent difference in the signal-sensitivity between DN and DP cells may in part reflect the distinct features of Rap and Ras signaling. It has been shown that Rap activation can persist for a long period depending on the duration and magnitude of receptor signals, whereas Ras activation is usually transient due to the tight negative feedback mechanisms.41 Because the rescue of pre-T cells from p53-dependent apoptosis is considered to be a time-consuming event,35 functional coupling of Rap activation with the sustained signal autonomously delivered by the properly assembled pre-TCR may ensure the survival of pre-T cells throughout the critical periods. Several reports reveal important roles of multiple small GTPases at distinct stages of a complex series of T-cell development.10 Our current results suggest that Rap signal plays an essential role in the early stage of β-selection by linking the expression of pre-TCR to the control of the p53-dependent checkpoint response in normal DN cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr D. L. Wiest for providing the SCID.adh cells, Dr M. Matsuda for the C3G-F plasmid, Dr J. Takeda for the Lck-Cre mice, Dr C. B. Wilson for the CD4-Cre mice, and Dr T. Kitamura for the PLAT-E cells and the pMCs-IG vector.
This work was supported by grants from the Ministry of Education, Culture, Science, Sports and Technology of Japan (N.M., M.H.).
Authorship
Contribution: N.M. and M.H. designed research; K.K., M.M., Y.N., Y.K., and S.-F.W. performed research; S.Y. and T.S. provided essential material; and N.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nagahiro Minato, Graduate School of Medicine, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan; e-mail: minato@imm.med.kyoto-u.ac.jp.
References
Author notes
*K.K., M.M., and Y.N. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal