Abstract
MicroRNAs (miRNAs), recently identified noncoding small RNAs, are emerging as key regulators in homeostasis of the immune system. Therefore, aberrant expression of miRNAs may be linked to immune dysfunction, such as in chronic inflammation and autoimmunity. In this study, we investigated the potential role of miRNAs in estrogen-mediated regulation of innate immune responses, as indicated by up-regulation of lipopolysaccharide (LPS)–induced interferon-gamma (IFNγ), inducible nitric oxide synthase (iNOS), and nitric oxide in splenic lymphocytes from estrogen-treated mice. We found that miR-146a, a negative regulator of Toll-like receptor (TLR) signaling, was decreased in freshly isolated splenic lymphocytes from estrogen-treated mice compared with placebo controls. Increasing the activity of miR-146a significantly inhibited LPS-induced IFNγ and iNOS expression in mouse splenic lymphocytes. Further, miRNA microarray and real-time reverse transcriptase–polymerase chain reaction (RT-PCR) analysis revealed that estrogen selectively up-regulates/down-regulates the expression of miRNAs in mouse splenic lymphocytes. miR-223, which is markedly enhanced by estrogen, regulates LPS-induced IFNγ, but not iNOS or nitric oxide in splenic lymphocytes. Inhibition of miR-223 activity decreased LPS-induced IFNγ in splenic lymphocytes from estrogen-treated mice. Our data are the first to demonstrate the selective regulation of miRNA expression in immune cells by estrogen and are indicative of an important role of miRNAs in estrogen-mediated immune regulation.
Introduction
The innate immune system is the first line of defense protecting the host from invasion by diverse microbial pathogens. To date, 13 members of the Toll-like receptor (TLR) family have been identified in mammalian cells, and each TLR recognizes and binds to specific microbial products called pathogen-associated molecular patterns (PAMPs).1,2 For example, TLR4 recognizes and binds to lipopolysaccharide (LPS), a gram-negative bacterial component, to trigger the myeloid differentiation primary-response protein 88 (MyD88)–dependent signaling pathway and/or the MyD88-independent signaling pathway, resulting in the production of inflammatory molecules such as type I interferon (IFN) and nitric oxide.1,3 While TLR-mediated inflammatory responses are important for controlling infections, overwhelming activation of TLR signaling is deleterious and can cause severe inflammatory disease. Thus, the activation of TLRs should be tightly regulated in vivo. Various mechanisms employed by different classes of negative regulators have been identified to regulate TLR triggered inflammatory immune responses.4,5 Recent publications indicate that microRNAs (miRNAs) fine-tune innate immune responses; thus, an entirely new paradigm of regulation of innate immunity is proposed.6,7
miRNAs are small (18-25 nucleotide long), noncoding RNAs that suppress gene expression at the posttranscriptional level by binding to the 3′UTR of target genes, resulting in either translation inhibition or mRNA degradation.8 Despite their recent identification, the impact of miRNAs on gene regulation is profound. miRNAs have been shown to be involved in the regulation of a variety of biologic processes including development, signal transduction, apoptosis, cell proliferation, and tumorigenesis.9-13 The role of miRNAs in normal immune function, as well as in inflammatory processes, is now emerging.7,14 The direct role of miRNAs in regulation of innate immune responses was first suggested by a study that indicated that miR-146 is a negative feedback regulator of TLR signaling.15 Additionally, a recent report indicates that miR-155 and miR-125b, which are induced and inhibited by LPS stimulation, respectively, have opposite effects on TNFα induction and may regulate endotoxin shock responses.16 Let-7i was shown to target the TLR4 receptor. Microbial infection decreased the expression of let-7i, which is associated with up-regulation of TLR4 in infected cholangiocytes.17 The direct role of miRNAs in innate immunity is further suggested by the function of miRNAs in combating viral infections.13,18 It was shown that IFNβ inhibits hepatitis C virus replication in the human hepatoma cell line Huh7 by inducing miRNAs that target the RNA genome of viruses.13
Estrogen, a sex hormone, has been well recognized as an important immune modulator and plays a central role in gender differences in disease susceptibility.19-21 Moreover, estrogen has been implicated in autoimmune diseases.22 Estrogen has also been shown to physiologically regulate the induction of inflammatory molecules by lymphoid cells. We, and others, have shown that in vivo estrogen treatment promotes induction of IFNγ, inducible nitric oxide synthase (iNOS), nitric oxide, monocyte chemoattractant protein-1 (MCP-1), and MCP-5.23-27 Estrogen may strengthen innate immune responses by promoting the differentiation of IFNγ-producing killer dendritic cells,28 or by up-regulation of iNOS expression and nitric oxide production.29 Further, estrogen has been shown to play a key role in controlling bacterial and viral infections in female mouse brains.30
In this novel report, we demonstrate that in vivo estrogen treatment significantly enhances innate immune responses of splenic lymphocytes to LPS by markedly augmenting induction of iNOS, nitric oxide, and IFNγ. Importantly, we show that LPS-induced IFNγ and iNOS are regulated by select miRNAs. Microarray and real-time reverse transcriptase–polymerase chain reaction (RT-PCR) analysis revealed that estrogen selectively up-regulates/down-regulates miRNA expression in splenic lymphocytes. By experimentally manipulating the activity of select estrogen-regulated miRNAs, we demonstrated that miR-146a and miR-223 regulate LPS-induced IFNγ in splenic lymphocytes. These data are the first to illustrate the mechanistic role of miRNAs in estrogen-mediated immune regulation and may have far-reaching implications in therapeutic treatment of estrogen-mediated immune disorders.
Methods
Mice and isolation of splenic lymphocytes
Wild-type C57BL/6 male mice (Charles River Laboratories, Wilmington, MA) were orchiectomized and surgically implanted with 17β-estradiol (Sigma-Aldrich, St Louis, MO) or empty (placebo control) silastic implants as extensively described in our previous studies.25,31-33 All animals were housed in the animal facility at the Center for Molecular Medicine and Infectious Diseases (CMMID). The Animal Care and Use Committee at Virginia Polytechnic Institute and State University approved all animal procedures. Mice were killed 7 to 8 weeks after implantation, and splenic lymphocytes were isolated and cultured using procedures previously described in detail.23,31 In brief, spleens from individual mice were dissociated in phenol red free RPMI-1640 incomplete medium (CellGro, Mediatech, Manassas, VA). Splenic lymphocytes were isolated with ACK-Tris-NH4Cl lysis buffer per our previous studies, and washed with complete RPMI-1640 that was supplemented with steroid-free 10% charcoal-stripped fetal bovine serum (Atlanta Biologicals, Norcross, GA), 2 mM l-glutamine (Mediatech), 100 IU/mL penicillin (Mediatech), 100 μg/mL streptomycin (Mediatech), and 1% nonessential amino acids (Mediatech). Splenic lymphocytes were resuspended in complete medium and adjusted to 5 × 106/mL before plating the cells.
Real-time RT-PCR
Total RNA, containing miRNAs, was isolated from freshly isolated splenic lymphocytes using mirVana miRNA isolation kits (Ambion, Austin, TX). The Taqman miRNA assay system (Applied Biosystems, Foster City, CA) was used to quantitatively detect the expression of miRNAs following the manufacturer's instructions. The relative expression level of miRNA was calculated using the 2−ΔΔCt (Livak) method after normalization to the endogenous small RNA control, snoRNA 202. To analyze the expression of IFNγ and iNOS mRNA, real-time RT-PCR was performed using iScript one-step RT-PCR kits with SYBR green (Bio-Rad, Hercules, CA) per our previously reported study.33 Total RNA was treated with RNAase-free DNAase (Promega, Madison, WI) and then used as a template. The mRNA expression level of target genes was normalized to β-actin using the 2−ΔΔCt (Livak) method. PCR primer mixes (10 ×) for IFNγ, iNOS, and β-actin were purchased from QIAGEN (Valencia, CA).
miRNA microarray assay and data analysis
Total RNA was isolated from freshly isolated splenic lymphocytes from placebo- and estrogen-treated mice (n = 2 each group) as described in “Real-time RT-PCR.” Small RNA enrichment and miRNA microarray assays were performed by LC Sciences (Houston, TX). Mouse miRNA array chips (Chip ID miRMouse 10.0 version), which included 568 unique, mature, mouse miRNA, based on the Sanger miRBase Release 10.0, were used in the assay. Each miRNA probe was repeated in sextuplicate on the chip. The data extracted from the image of each hybridized chip were adjusted by subtracting the background signal (the median of 5% to 25% of the lower signal intensities). Normalization was further carried out using the Variance Stabilization Normalization (VSN) method on background-subtracted data to remove system-related variations.34 The means of the normalized signal intensities from the 6 signal spots for each probe were Log2 transformed for further analysis. To visualize the differential expression intensity levels of miRNAs in different samples, a heat map was generated using Java Tree View.35 The Log2-transformed intensity values were centered by subtracting the mean Log2 values across all the samples for individual miRNAs, and then used for cluster analysis to generate the heat map. The significance of miRNAs differentially expressed between the 2 groups of samples (placebo- vs estrogen-treated mice) was determined using the LIMMA package36 . The raw P values were further corrected by the Benjamini and Hochberg false-discovery rate test.37 The fold-change was calculated by dividing the mean intensity of the miRNAs in estrogen-treated samples by that in placebo-treated samples. If this number was less than one, the negative reciprocal was used. The microarray data set has been submitted to Gene Expression Omnibus (GEO) under the accession number GSE11197.
Transfection of miRIDIAN miRNA mimics and inhibitors
miRIDIAN miRNA mimics (double-stranded chemically modified RNA oligonucleotides) and miRIDIAN miRNA inhibitors (single-stranded chemically enhanced oligonucleotides) from Dharmacon RNA Technologies (Lafayette, CO) were used to supplement and suppress specific miRNA activity in mouse splenic lymphocytes, respectively. Both a nucleofector device and mouse macrophage nucleofector kit (Amaxa Biosystems, Gaithersburg, MD) were used to transfect 3.5 μg miRNA mimics or inhibitors to 1.5 × 107 freshly isolated mouse splenic lymphocytes. Negative miRIDIAN mimics or inhibitors were transfected as matched controls.
Detection of iNOS, nitric oxide, and IFNγ
Western blots were used to analyze iNOS and IFNγ protein expression in whole cell extracts as described before.23,33 The blot images were captured and the signal intensities were analyzed using a Kodak Image Station 440. Griess assays were used to detect nitric oxide levels in culture supernatants as described.23 The levels of IFNγ in culture supernatants were determined with enzyme-linked immunosorbent assays (ELISAs) as previously described.24,31
Statistical analysis
All values in the graphs are given as means plus or minus standard error of the mean (SEM). To assess statistical significance, t tests were performed using GraphPad InStat version 3.0a for Macintosh (GraphPad Software, San Diego, CA). For evaluation of the effect of a specific miRNA mimic or inhibitor on LPS-induced nitric oxide and IFNγ, the levels of nitrite and IFNγ in negative control transfected cells were regarded as 100% and the levels of nitrite and IFNγ in paired miRNA mimic- or inhibitor-transfected cells are shown as a percentage of the level of the corresponding negative control transfected cells. Paired t tests were performed between negative control and specific inhibitor or mimic transfected cells.
Results
LPS-induced IFNγ is augmented in splenic lymphocytes by estrogen
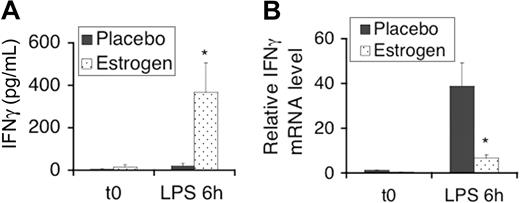
Our previous report has shown that estrogen promotes secretion of the proinflammatory cytokine IFNγ in splenic lymphocytes after stimulation with Con-A for only 3 hours.38 In the present study, we activated splenic lymphocytes with LPS and then determined the IFNγ level in culture supernatants. At 3 hours of stimulation with LPS, the IFNγ levels in supernatants of splenic lymphocytes were low (undetected in some samples), and thus, there was no significant difference between placebo- and estrogen-treated mice (data not shown). After 6 hours of stimulation with LPS, however, we observed significantly higher IFNγ levels in supernatants of splenic lymphocytes from estrogen-treated mice compared with placebo-treated (control) mice (Figure 1A). LPS stimulation induced IFNγ mRNA expression in splenic lymphocytes from both placebo- and estrogen-treated mice. Intriguingly, in contrast to the increased IFNγ protein levels in the supernatants of LPS-stimulated splenic lymphocytes from estrogen-treated mice, the level of IFNγ mRNA was significantly lower than that from placebo-treated mice (Figure 1B). This suggested that the expression of LPS-induced IFNγ in splenocytes from estrogen-treated mice is regulated at both transcriptional and posttranscriptional levels.
LPS-induced IFNγ in mouse splenic lymphocytes is promoted by estrogen at the posttranscriptional level. (A) ELISA analysis of IFNγ levels in supernatants from 5 × 106 freshly isolated (t0) splenocytes and those stimulated with LPS (500 ng/mL, the same concentration was used in all the experiments in this study) for 6 hours. The graphs show the means plus or minus SEM (n = 4 each). (B) Total RNA was prepared from freshly isolated splenocytes and from those stimulated with LPS for 6 hours. IFNγ mRNA expression levels were determined by real-time RT-PCR. The graphs show the relative mRNA expression levels with the means plus or minus SEM (n ≥ 3). *P < .05.
LPS-induced IFNγ in mouse splenic lymphocytes is promoted by estrogen at the posttranscriptional level. (A) ELISA analysis of IFNγ levels in supernatants from 5 × 106 freshly isolated (t0) splenocytes and those stimulated with LPS (500 ng/mL, the same concentration was used in all the experiments in this study) for 6 hours. The graphs show the means plus or minus SEM (n = 4 each). (B) Total RNA was prepared from freshly isolated splenocytes and from those stimulated with LPS for 6 hours. IFNγ mRNA expression levels were determined by real-time RT-PCR. The graphs show the relative mRNA expression levels with the means plus or minus SEM (n ≥ 3). *P < .05.
In vivo estrogen treatment decreases the expression of miR-146a in splenic lymphocytes
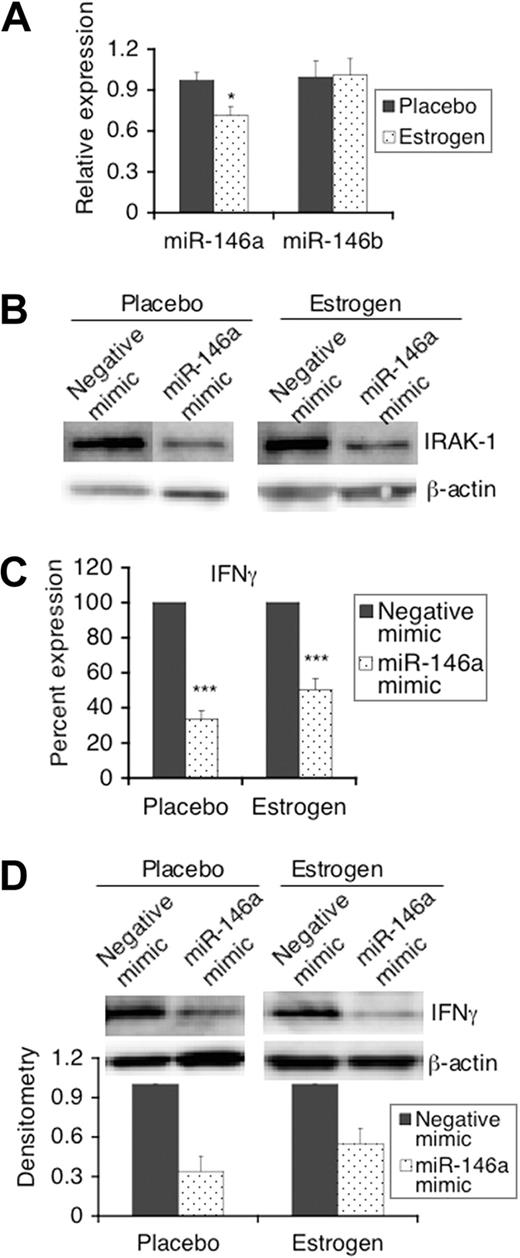
Given a recent report indicating the negative regulatory role of miR-146 in TLR signaling in human THP-1 monocytes,15 we next investigated whether miR-146 plays a similar role in mouse splenic lymphocytes and whether estrogen modulates the response to LPS by altering the expression of miR-146. Quantitative real-time RT-PCR analysis indicated that the expression of miR-146a is significantly decreased in freshly isolated splenic lymphocytes from estrogen-treated mice compared with controls (Figure 2A). In contrast to down-regulation of miR-146a levels, estrogen has no apparent effect on miR-146b since the levels of miR-146b were comparable in freshly isolated splenic lymphocytes from placebo- and estrogen-treated mice (Figure 2A).
LPS-induced IFNγ in mouse splenic lymphocytes is regulated by the estrogen-regulated miRNA, miR-146a. (A) Total RNA was isolated from freshly isolated splenic lymphocytes using mirVana miRNA isolation kits. Relative expression levels of miR-146a and miR-146b between splenic lymphocytes from placebo- and estrogen-treated mice were analyzed using the Taqman miRNA assay system. The graph shows the means plus or minus SEM (n ≥ 6 each). (B-D) Freshly isolated splenic lymphocytes (1.5 × 107) from placebo- and estrogen-treated mice were transfected with either a negative mimic (control) or miR-146a mimic. Twenty-four hours after transfection, cells were left unstimulated (B) or stimulated with LPS for 24 hours (C,D), and then the supernatants and cell pellets were collected for analysis. (B) Western blot analysis of the expression of IRAK-1 in unstimulated cells at 48 hours after transfection. (C) The level of IFNγ in supernatants from LPS-stimulated miR-146a mimic transfected cells is shown as the percentage expression of negative control mimic transfected cells. Graph shows mean plus or minus SEM (n ≥ 6 each). (D) Western blot analysis of the expression of IFNγ in cell extracts from negative mimic and miR-146a mimic transfected cells stimulated with LPS. Representative Western blot images are shown from at least 3 independent experiments. Densitometry analysis of the IFNγ signal in blot images was performed using Kodak molecular imaging software (version 4.5) and normalized to the loading control β-actin. The graph shows relative density with means plus or minus SEM (n = 3 each). *P < .05; ***P < .001.
LPS-induced IFNγ in mouse splenic lymphocytes is regulated by the estrogen-regulated miRNA, miR-146a. (A) Total RNA was isolated from freshly isolated splenic lymphocytes using mirVana miRNA isolation kits. Relative expression levels of miR-146a and miR-146b between splenic lymphocytes from placebo- and estrogen-treated mice were analyzed using the Taqman miRNA assay system. The graph shows the means plus or minus SEM (n ≥ 6 each). (B-D) Freshly isolated splenic lymphocytes (1.5 × 107) from placebo- and estrogen-treated mice were transfected with either a negative mimic (control) or miR-146a mimic. Twenty-four hours after transfection, cells were left unstimulated (B) or stimulated with LPS for 24 hours (C,D), and then the supernatants and cell pellets were collected for analysis. (B) Western blot analysis of the expression of IRAK-1 in unstimulated cells at 48 hours after transfection. (C) The level of IFNγ in supernatants from LPS-stimulated miR-146a mimic transfected cells is shown as the percentage expression of negative control mimic transfected cells. Graph shows mean plus or minus SEM (n ≥ 6 each). (D) Western blot analysis of the expression of IFNγ in cell extracts from negative mimic and miR-146a mimic transfected cells stimulated with LPS. Representative Western blot images are shown from at least 3 independent experiments. Densitometry analysis of the IFNγ signal in blot images was performed using Kodak molecular imaging software (version 4.5) and normalized to the loading control β-actin. The graph shows relative density with means plus or minus SEM (n = 3 each). *P < .05; ***P < .001.
miR-146a down-regulates LPS-induced IFNγ in mouse splenic lymphocytes
Next, we further investigated whether miR-146a regulates LPS-induced IFNγ in mouse splenic lymphocytes by manipulating its activity using miR-146a mimics, which can supplement the activity of miR-146a in vivo. As indicated, in the cells transfected with miR-146a, the expression of IRAK-1, a confirmed miR-146 target, was decreased compared with that in negative mimic transfected cells (Figure 2B). With enhanced miR-146a activity, there was a significant decrease in LPS-induced IFNγ cytokine levels in culture supernatants from both placebo- and estrogen-treated mice when compared with negative mimic control transfected cells (Figure 2C). Moreover, Western blotting clearly showed that IFNγ protein expression in cells transfected with miR-146a mimics was decreased compared with negative mimic transfected cells (Figure 2D). Together, the data indicated that miR-146a negatively regulates LPS-induced IFNγ in mouse splenic lymphocytes.
LPS-induced iNOS and nitric oxide in mouse splenic lymphocytes are regulated by miR-146a
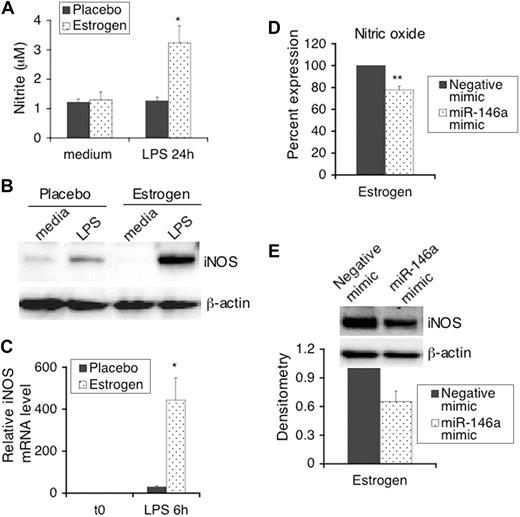
In our previous studies we reported that estrogen markedly enhanced IFNγ-dependent induction of iNOS and nitric oxide in splenic lymphocytes that were activated with Con-A for 24 hours.23 In this study, we reported that LPS activated splenic lymphocytes from estrogen-treated mice had also enhanced nitric oxide (Figure 3A) and iNOS protein expression (Figure 3B). Consistent with increased iNOS protein expression, iNOS mRNA is markedly higher in splenic lymphocytes from estrogen-treated mice compared with placebo controls (Figure 3C). This suggested that estrogen promotes LPS-induced iNOS at the transcriptional level. Further, we also determined whether increasing the function of miR-146a down-regulates LPS-induced iNOS and nitric oxide in splenic lymphocytes from estrogen-treated mice. There was a significant decrease in nitric oxide in culture supernatants from miR-146a mimic transfected splenic lymphocytes compared with negative mimic transfected cells (Figure 3D, P < .01). Further, Western blot analysis also showed decreased iNOS protein expression in cells transfected with miR-146a mimics (Figure 3E). However, the down-regulatory effect of the miR-146a mimics on nitric oxide was not as strong as that observed for LPS-induced IFNγ. Since the levels of LPS-induced nitric oxide in splenic lymphocytes from placebo-treated mice were too low, down-regulatory effects of miR-146a mimics on nitric oxide in cells from placebo-treated mice could not be fully determined. However, we did observe that the miR-146a mimics inhibited LPS-induced iNOS protein expression in some samples from placebo mice in which iNOS could be detected by Western blotting (data not shown). Our data indicate that estrogen promotes LPS-induced iNOS and nitric oxide, which are partially regulated by the activity of miR-146a.
LPS-induced iNOS and nitric oxide in mouse splenic lymphocytes is enhanced by estrogen and regulated by miR-146a. An aliquot of 2.5 × 106 splenic lymphocytes (5 × 106/mL) from placebo- and estrogen-treated mice (n = 4 each) were stimulated with LPS or left unstimulated for 24 hours (medium only; A,B). Supernatants and cell pellets were collected for further analysis. (A) The production of nitric oxide in culture supernatants was determined with Griess assays. The graph shows means plus or minus SEM (n = 4 each). (B) Western blot analysis of the expression of iNOS in whole cell extracts. (C) The expression of iNOS mRNA in freshly isolated and 6 hours of LPS stimulated spenic lymphocytes was analyzed as indicated for Figure 1B. The graphs show the relative mRNA expression level with the means plus or minus SEM (n ≥ 3 each). (D) Freshly isolated splenic lymphocytes were transfected and stimulated with LPS as described for Figure 2B-D. The level of nitric oxide in supernatants from LPS-stimulated transfected cells was determined with Greiss assays. The graph shows means plus or minus SEM (n ≥ 6 each). (E) Western blot analysis of the expression of iNOS in cell extracts from negative mimic and miR-146a mimic transfected cells stimulated with LPS. Representative Western blot images are shown from at least 3 independent experiments. Densitometry analysis of iNOS signal detected by Western blotting was performed using Kodak molecular imaging software (version 4.5) and normalized to the loading control β-actin. The graph shows relative density with means plus or minus SEM (n = 4). *P < .05; **P < .01.
LPS-induced iNOS and nitric oxide in mouse splenic lymphocytes is enhanced by estrogen and regulated by miR-146a. An aliquot of 2.5 × 106 splenic lymphocytes (5 × 106/mL) from placebo- and estrogen-treated mice (n = 4 each) were stimulated with LPS or left unstimulated for 24 hours (medium only; A,B). Supernatants and cell pellets were collected for further analysis. (A) The production of nitric oxide in culture supernatants was determined with Griess assays. The graph shows means plus or minus SEM (n = 4 each). (B) Western blot analysis of the expression of iNOS in whole cell extracts. (C) The expression of iNOS mRNA in freshly isolated and 6 hours of LPS stimulated spenic lymphocytes was analyzed as indicated for Figure 1B. The graphs show the relative mRNA expression level with the means plus or minus SEM (n ≥ 3 each). (D) Freshly isolated splenic lymphocytes were transfected and stimulated with LPS as described for Figure 2B-D. The level of nitric oxide in supernatants from LPS-stimulated transfected cells was determined with Greiss assays. The graph shows means plus or minus SEM (n ≥ 6 each). (E) Western blot analysis of the expression of iNOS in cell extracts from negative mimic and miR-146a mimic transfected cells stimulated with LPS. Representative Western blot images are shown from at least 3 independent experiments. Densitometry analysis of iNOS signal detected by Western blotting was performed using Kodak molecular imaging software (version 4.5) and normalized to the loading control β-actin. The graph shows relative density with means plus or minus SEM (n = 4). *P < .05; **P < .01.
miRNA expression profiles in mouse splenic lymphocytes are modulated by estrogen
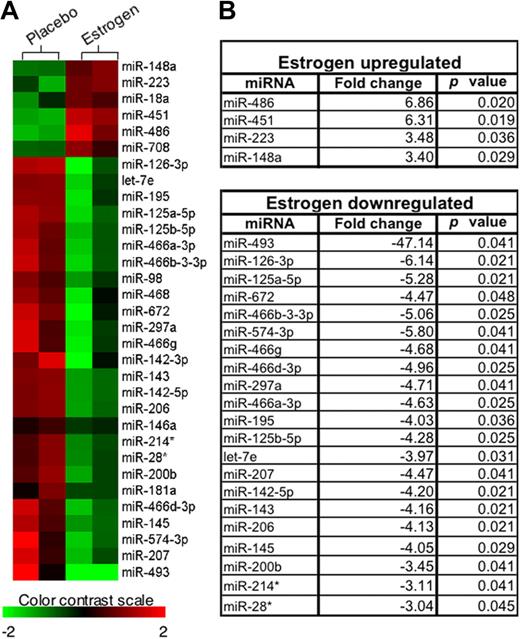
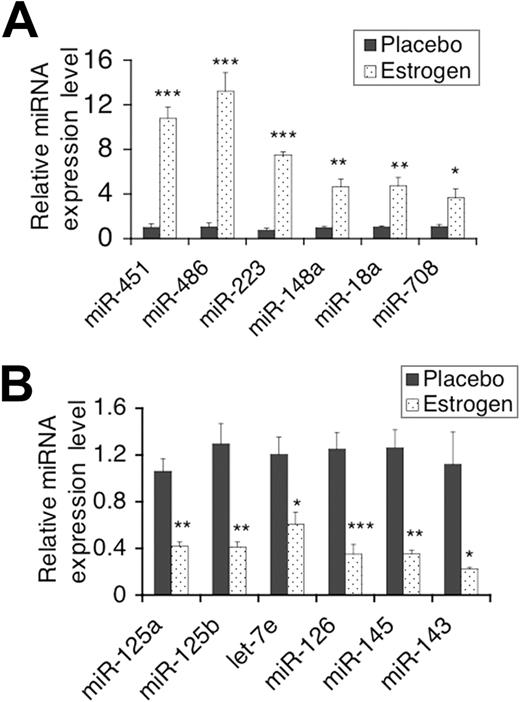
The above data suggested that estrogen regulates miR-146a, which in turn regulates splenic lymphocyte responses to LPS. It is therefore likely that estrogen may also regulate other miRNAs in splenic lymphocytes, an aspect not yet determined. We performed miRNA microarray assays to compare the miRNA expression profiles in freshly isolated splenic lymphocytes from estrogen-treated mice to those from placebo-treated mice (n = 2 per group). The heat map, which was generated using hierarchical cluster analysis, revealed that the expression pattern of miRNAs in freshly isolated splenic lymphocytes from estrogen-treated mice is distinct from that observed in placebo (control)–treated mice (Figure 4A). In Figure 4B, miRNAs that were significantly different between placebo- and estrogen-treated mice are listed, based on statistical analysis of the microarray data (P < .05). Estrogen up-regulated several miRNAs (eg, miR-451, miR-223) as depicted by the shade of red in the heat map and by the statistical analysis (Figure 4A,B). Estrogen also significantly down-regulated miRNAs (eg, miR-145 and miR-125a), which are shown as shades of green in the heat map (P < .05; Figure 4A,B). We next confirmed changes in selected differentially expressed miRNAs using real-time RT-PCR with increased sample sizes (n = 4-6 per treatment group). Real-time RT-PCR analysis confirmed that estrogen significantly increased the expression of miR-451, miR-486, miR-223, and miR-148a compared with samples from placebo-treated mice (Figure 5A). Conversely, real-time RT-PCR results indicated that estrogen also significantly decreased the expression of miR-125a-5p (miR-125a), miR-125b-5p (miR-125b), miR-143, miR-145, let-7e, and miR-126-3p (miR-126; Figure 5B). Additionally, real-time PCR revealed that miR-18a and miR-708, which were increased, but not statistically significant based on the microarray data, were also significantly up-regulated by estrogen.
Microarray data analysis. (A) The heat map was generated using hierarchical cluster analysis to show distinct miRNA expression patterns in splenic lymphocytes between placebo- and estrogen-treated mice. The intensity values were Log2 transformed, centered by the mean of individual genes across all 4 samples, and then subjected to cluster analysis for generating the heat map. The color bar was extracted to show the color contrast level of the heat map. Red and green indicate high expression level and low expression level, respectively. (B) miRNAs that demonstrated statistically different expression levels between freshly isolated splenic lymphocytes from placebo- and estrogen-treated mice (P < .05) are listed. Fold-changes in miRNA expression were calculated as the ratio of mean intensity values between estrogen- and placebo-treated mice.
Microarray data analysis. (A) The heat map was generated using hierarchical cluster analysis to show distinct miRNA expression patterns in splenic lymphocytes between placebo- and estrogen-treated mice. The intensity values were Log2 transformed, centered by the mean of individual genes across all 4 samples, and then subjected to cluster analysis for generating the heat map. The color bar was extracted to show the color contrast level of the heat map. Red and green indicate high expression level and low expression level, respectively. (B) miRNAs that demonstrated statistically different expression levels between freshly isolated splenic lymphocytes from placebo- and estrogen-treated mice (P < .05) are listed. Fold-changes in miRNA expression were calculated as the ratio of mean intensity values between estrogen- and placebo-treated mice.
Real-time RT-PCR analysis of miRNA expression in splenic lymphocytes. The expression level of selected estrogen-regulated miRNA in freshly isolated splenic lymphocytes between placebo- and estrogen-treated mice were further quantified using Taqman miRNA assays. (A,B) The graphs show miRNA that were confirmed to be significantly up-regulated or down-regulated by estrogen treatment, respectively. Means plus or minus SEM (n ≥ 4 each) are shown in the graphs. *P < .05; **P < .01; and ***P < .001.
Real-time RT-PCR analysis of miRNA expression in splenic lymphocytes. The expression level of selected estrogen-regulated miRNA in freshly isolated splenic lymphocytes between placebo- and estrogen-treated mice were further quantified using Taqman miRNA assays. (A,B) The graphs show miRNA that were confirmed to be significantly up-regulated or down-regulated by estrogen treatment, respectively. Means plus or minus SEM (n ≥ 4 each) are shown in the graphs. *P < .05; **P < .01; and ***P < .001.
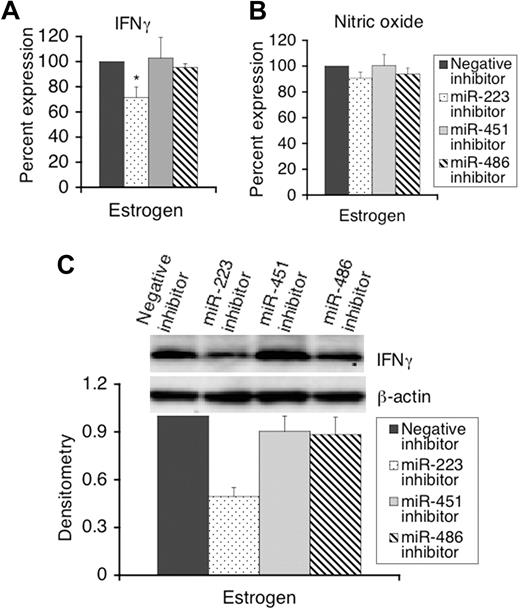
Inhibition of miR-223 activity leads to a reduction of LPS-induced IFNγ
Since estrogen highly up-regulates the expression of miR-451, miR-486, and miR-223 (over 8-fold based on the real-time RT-PCR results) in splenic lymphocytes, we further investigated whether these 3 miRNAs play roles in estrogen-mediated enhancement of LPS-induced IFNγ and iNOS expression. We inhibited the function of miR-223, miR-486, and miR-223 using appropriate inhibitors, and then determined the expression of LPS-induced IFNγ, iNOS, and nitric oxide. Inhibiting the activity of miR-223, but not miR-451 and miR-486, significantly decreased LPS-induced IFNγ secretion in supernatants of splenic lymphocytes from estrogen-treated mice compared with negative transfected cells (Figure 6A). However, none of 3 miRNAs had significant effects on nitric oxide production in culture supernatants since there was no significant change in the nitric oxide levels between negative inhibitor and specific inhibitor transfected cells (Figure 6B). Further, Western blot analysis indicated that IFNγ protein expression is decreased in miR-223 inhibitor, but not in miR-451 and miR-486 inhibitor, transfected cells, compared with negative controls (Figure 6C).
miR-223, but not miR-451 and miR-486, regulate LPS-induced IFNγ in splenic lymphocytes from estrogen-treated mice. Freshly isolated splenic lymphocytes (1.5 × 107) from estrogen-treated mice were transfected with either a negative inhibitor (control), miR-223, miR-451, or miR-486 inhibitors. Twenty-four hours after transfection, cells were stimulated with LPS for 24 hours, and the supernatants and cell pellets were collected for analysis. (A,B) The level of IFNγ and nitric oxide in culture supernatants of LPS-stimulated cells were determined by ELISAs (A) and Griess assays (B), respectively. The level of IFNγ and nitric oxide in supernatants from specific inhibitor transfected cells were presented as the percentage level of negative control inhibitor transfected cells. The graphs show the means plus or minus SEM (n = 5 each). (C) Western blot analysis of the expression of IFNγ protein in miRNA inhibitor transfected cells. Representative Western images are shown from at least 3 independent experiments. Densitometry analysis of IFNγ signal was performed as described for Figure 2. The graph shows relative density with means plus or minus SEM (n = 4 each). *P < .05.
miR-223, but not miR-451 and miR-486, regulate LPS-induced IFNγ in splenic lymphocytes from estrogen-treated mice. Freshly isolated splenic lymphocytes (1.5 × 107) from estrogen-treated mice were transfected with either a negative inhibitor (control), miR-223, miR-451, or miR-486 inhibitors. Twenty-four hours after transfection, cells were stimulated with LPS for 24 hours, and the supernatants and cell pellets were collected for analysis. (A,B) The level of IFNγ and nitric oxide in culture supernatants of LPS-stimulated cells were determined by ELISAs (A) and Griess assays (B), respectively. The level of IFNγ and nitric oxide in supernatants from specific inhibitor transfected cells were presented as the percentage level of negative control inhibitor transfected cells. The graphs show the means plus or minus SEM (n = 5 each). (C) Western blot analysis of the expression of IFNγ protein in miRNA inhibitor transfected cells. Representative Western images are shown from at least 3 independent experiments. Densitometry analysis of IFNγ signal was performed as described for Figure 2. The graph shows relative density with means plus or minus SEM (n = 4 each). *P < .05.
Discussion
In this study, we demonstrated that increasing the activity of miR-146a in vivo by transfecting cells with miR-146a mimics significantly decreased LPS-induced IFNγ in both splenic lymphocytes from placebo- and estrogen-treated mice. Our data also provide direct evidence showing that miR-146a targets IRAK-1 and negatively regulates LPS-TLR4–mediated inflammatory responses in splenic lymphocytes. Further, we found that in vivo estrogen treatment decreased the expression of miR-146a in freshly isolated splenic lymphocytes compared with placebo controls. It is therefore conceivable that the lower level of miR-146a in freshly isolated cells allows earlier induction of IFNγ in splenic lymphocytes from estrogen-treated mice (6 hours).
Enhancing the activity of miR-146a also inhibited the expression of LPS-induced iNOS and nitric oxide, which are markedly augmented in splenic lymphocytes from estrogen-treated mice. It is notable that the inhibitory effect of miR-146a on LPS-induced IFNγ (about 50%, Figure 2C) is stronger than that observed for LPS-induced nitric oxide (about 20%, Figure 3C). One possibility is that LPS-induced IFNγ and iNOS are subjected to different regulatory machinery downstream of LPS-TLR signaling. LPS-induced type I interferon and IFNγ can prime the cells and further synergize with LPS to stimulate maximal production of nitric oxide in macrophages.39,40 Additionally, we noticed that estrogen enhances the expression of iNOS at both the mRNA and protein levels. However, LPS-induced IFNγ mRNA levels are significantly lower in splenic lymphocytes from estrogen-treated mice after LPS stimulation compared with placebo controls (Figure 1B). These data suggest that there is a different underlying mechanism in estrogen-mediated promotion of LPS-induced IFNγ compared with LPS-induced iNOS/nitric oxide. It is possible that estrogen regulates LPS-induced IFNγ at the posttranscriptional level by down-regulation of IFNγ targeting miRNAs. Identification of IFNγ-targeting miRNAs may further delineate whether estrogen regulates IFNγ at the posttranscriptional level by regulating IFNγ-targeting miRNAs.
Although limited, recent reports have suggested hormonal regulation of miRNAs in nonlymphoid models, such as rat mammary glands and zebrafish.41,42 However, so far there are no reports regarding hormonal regulation of miRNAs in immune cells. Based on the microarray analysis, 25 miRNAs were significantly regulated by estrogen in splenic lymphocytes (P < .05, Figure 4B). Selected miRNAs were further confirmed using real-time RT-PCR analysis (Figure 5). Additionally, estrogen significantly decreased miR-146a, and increased miR-18a and miR-708 as determined by real-time RT-PCR (Figures 2A and 5A), but not by microarray analysis. The discrepancy in statistical differences between real-time RT-PCR and microarray analysis (regarding the detection of miR-146a, miR-18a, and miR-708) is most likely due to the difference in the sensitivity of these 2 assays, biologic differences, and the fact that unlike real-time RT-PCR, a limited number of samples (n = 2) were used in the microarray analysis. Moreover, although the miRNA microarray is an efficient and sensitive screening tool for simultaneous analysis of the expression of multiple miRNAs, it can also result in false positives/negatives.43,44 Therefore, confirmation of microarray data using real-time RT-PCR or Northern blotting is a necessity. Here, we confirmed the changes of selected miRNAs by real-time RT-PCR with an increased sample size (n = 4-6).
Intriguingly, almost all of the miRNAs confirmed in our studies to be down-regulated by estrogen (miR-125a, miR-125b, miR-143, miR-145, let-7, miR-126) are decreased or lost in breast cancer.45,46 The decreased expression of these breast cancer–related miRNAs by estrogen treatment may provide an explanation for increasing breast cancer risk following lengthy exposure to estrogen, such as with postmenopausal hormone replacement therapy.47,48
miR-223 has been shown to regulate granulocyte inflammatory responses.49 Here, we found that inhibiting the activity of miR-223 decreased LPS-induced IFNγ in splenic lymphocytes from estrogen-treated mice. Another 2 miRNAs highly up-regulated by estrogen, miR-451 and miR-486, had no significant effect on LPS-induced IFNγ and nitric oxide (Figure 6). It is possible that miR-451 and miR-486 are not involved in LPS signaling. We believe that there are multiple miRNAs, not single miRNA, which cooperate with each other in the regulation of innate immune responses. Given that estrogen up-/down-regulates a panel of miRNAs, simultaneous inhibition and/or enhancement of the activity of several estrogen-regulated miRNAs may result in more significant changes in LPS-induced inflammatory molecules compared with negative control transfected cells.
In this study, we used red blood cell (RBC)–depleted splenic lymphocytes as model cells to investigate the role of miRNAs in estrogen-mediated regulation of inflammatory responses since in a natural in situ setting, cell-cell interactions are critical for regulating the outcome of immune responses. This aspect was highlighted in our previous studies, which showed that Con-A–induced IFNγ and nitric oxide can be dramatically decreased in splenic lymphocytes from both placebo- and estrogen-treated mice by blocking binding of the CD28 receptor on T cells with B7 molecules on antigen-presenting cells.23 Here, we used a different approach by using purified subsets of cells to show that the induction of IFNγ after 6 hours of LPS stimulation and the induction of nitric oxide levels after 48 hours of LPS stimulation are very low in purified macrophages, T cells, or macrophage-depleted splenic lymphocytes from estrogen-treated mice. However, there is dramatic induction of IFNγ and nitric oxide when purified macrophages and T cells are mixed together before LPS stimulation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These data reinforced the growing belief that splenic lymphocytes simulate the natural conditions where APCs, T cells, and B cells interact and influence each other to regulate immune responses. Subsequent studies can potentially focus on addressing a separate question of whether estrogen differentially regulates miRNAs in defined subpopulations of splenic lymphocytes.
The present report is novel because it is the first study to demonstrate that estrogen selectively regulates miRNA expression in immune cells. Further, we demonstrate that select estrogen-regulated miRNAs, miR-146a and miR-223, regulated LPS-induced IFNγ, and to a lesser extent nitric oxide, in splenic lymphocytes. These data suggest a novel mechanism for estrogen regulation of the immune system. Further investigation of biologic and immunologic functions of estrogen-regulated miRNAs is important to allow comprehensive delineation of the role of estrogen-regulated miRNAs in both normal and dysregulated immune systems. Given that estrogens are thought to be involved in not only normal immune regulation, but also in many inflammatory disease conditions, these studies may have profound implications and may offer an entirely new molecular therapeutics approach for treatment of estrogen-related immune disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ebru Karpuzoglu and Mr Carmine Granielo for collecting samples, preparing implants, and assisting with surgeries. We also thank Mr Peter Jobst, Mr Dustin Lucas, and the animal care staff.
This work was supported by grants from the National Institutes of Health (1 RO1 AI051880-05) and the Virginia-Maryland Regional College of Veterinary Medicine (VMRCVM) Intramural Research Competition (IRC) Grant (441303) to S.A.A. The microarray data analysis by Y.Z. and O.C. was funded through the CRT funding from Virginia Bioinformatics Institute to O.C.
National Institutes of Health
Authorship
Contribution: R.D. and S.A.A. designed and performed the experiments, analyzed data, and prepared the manuscript; R.A.P. and D.K. performed experiments and contributed to paper writing; and Y.Z. and O.C. contributed to microarray experiment design and data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Ansar Ahmed, Center for Molecular Medicine and Infectious Diseases, Department of Biomedical Sciences and Pathobiology, Virginia-Maryland Regional College of Veterinary Medicine, Virginia Tech, 1410 Prices Fork Road, Blacksburg, VA 24061-0342; e-mail: ansrahmd@vt.edu.
References
Author notes
*R.A.P. and Y.Z. contributed equally to this work.