To the editor:
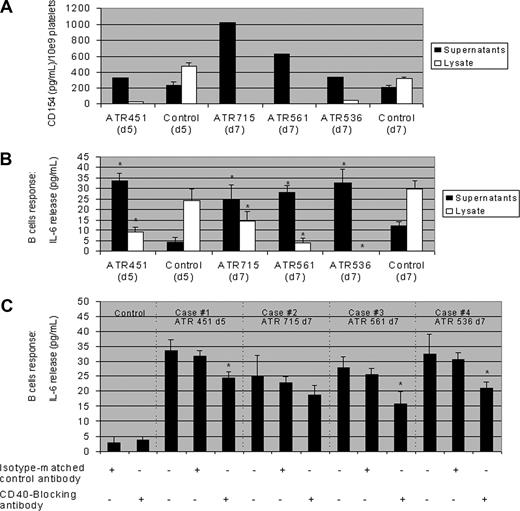
Several independent studies indicate that soluble CD40 ligand (sCD40L) derived and cleaved from platelets is responsible for acute transfusion reactions (ATR).1-3 Ratliff et al4,5 show in this journal that platelets modulate innate and adaptive immunity in mice away from the site of activation and impact antibody-mediated immune responses. Having shown that platelet-derived sCD40L alters human B-cell responses in vitro,6 we examined whether sCD40L in platelet concentrates (PCs) associated with clinical ATR could mediate B-cell responses as an indication of pathophysiologic function. Apheresed PCs were collected and processed with leukocyte reduction (< 106 per unit); suspended in 35% donor plasma and 65% InterSol platelet additive solution (Fenwal, La Chatre, France); prepared with the amotosalen HCl plus UVA light pathogen inactivation procedure (Intercept; Cerus, Concord, CA); and stored at 22°C with shaking for 5 or 7 days before transfusion.7 An active hemovigilance program evaluated the response to platelet transfusion.7 Reported ATR episodes were investigated using residual platelet components associated with ATR. In the 4 investigated cases of ATR (PCs were older than 3 days; Figure 1),8 2 aliquots from each PC (and, for each aliquot, 10 controls not associated with ATR) were prepared. One aliquot was used to assay supernatant fractions and the other to assay platelet lysates using specific, sensitive ELISAs (R&D Systems Europe, Lille, France) targeting a panel of cytokines and chemokines. IL8, CD62p, and platelet-derived growth factor–AB (PDGF-AB) levels were similar between ATR-associated PCs and PCs without ATR. In ATR-associated PCs, supernatant fractions contained higher levels of sCD40L than the control component, consistent with release; in an inverse correlation, the corresponding platelet lysates contained lower levels of sCD40L, consistent with release during storage (P < .05). To determine whether the released sCD40L (possibly among other costimulators) was biologically active, we incubated purified B cells, isolated from the blood of healthy donors, with PC supernatants and platelet lysates from PCs either associated or not with ATR. We then measured B-cell production of IL-6, on day 2 of the culture, to identify a production plateau (F.C., unpublished data, April 6, 2006).
Concentration and physiologic effects of soluble CD40 ligand (sCD40L) in supernatants and lysates of platelet concentrates (PCs) associated with ATR(451,715,561,536) and control PCs. (A) Determination of sCD40L levels in PC supernatants and lysates in platelet components associated with ATR versus control components; data obtained from the clinical ATR occurrence with a day-5 (d5) PC sample and a d7 PC sample. (B) PC supernatants and lysates of individual components associated with reactions were tested with purified blood B cells from healthy blood donors stimulated to secrete IL-6; data obtained from the clinical episodes with one d5 and 3 d7 PC samples, respectively. (C) The consistent decrease of IL-6 secretion in response to stimulation with a high concentration of sCD40L in platelet supernatants after preincubation of B cells with 5 μg/mL CD40-blocking antibody. We observed similar results by preincubation of B cells with 5 μg/mL of another CD40-blocking antibody and with both antibodies together (data not shown). All values (pg/mL) were corrected for background levels. Data are expressed as means plus or minus SD in n = 5 experiments. *P < .05 in differences between tested values and respective controls (Wilcoxon paired test).
Concentration and physiologic effects of soluble CD40 ligand (sCD40L) in supernatants and lysates of platelet concentrates (PCs) associated with ATR(451,715,561,536) and control PCs. (A) Determination of sCD40L levels in PC supernatants and lysates in platelet components associated with ATR versus control components; data obtained from the clinical ATR occurrence with a day-5 (d5) PC sample and a d7 PC sample. (B) PC supernatants and lysates of individual components associated with reactions were tested with purified blood B cells from healthy blood donors stimulated to secrete IL-6; data obtained from the clinical episodes with one d5 and 3 d7 PC samples, respectively. (C) The consistent decrease of IL-6 secretion in response to stimulation with a high concentration of sCD40L in platelet supernatants after preincubation of B cells with 5 μg/mL CD40-blocking antibody. We observed similar results by preincubation of B cells with 5 μg/mL of another CD40-blocking antibody and with both antibodies together (data not shown). All values (pg/mL) were corrected for background levels. Data are expressed as means plus or minus SD in n = 5 experiments. *P < .05 in differences between tested values and respective controls (Wilcoxon paired test).
Baseline IL-6 concentrations were consistently less than 5 to 10 pg/mL in each control. The addition of 20 μL 1/20 diluted “ATR” supernatant samples to 2 × 104 purified B cells in 200 μL culture medium9 resulted in increased IL-6 production compared with samples from control PCs (P < .05), the corresponding platelet lysates from ATR-associated PCs failed to elicit IL-6 production; recombinant purified sCD40L stimulated IL-6 production (P < .05), a cytokine strongly reactive to B cell stimulation. Preincubation of B cells with 5 μg/mL CD40-blocking antibodies (R&D Systems Europe and ATCC, Manassas, VA) substantially abrogated IL-6 secretion, unlike isotype-matched control. The partial blocking of CD40 binding on CD40+ B cells strongly suggests a potentially synergistic role in B cells for cytokines other than sCD40L (under investigation) and indicates a sustained role for PC-derived sCD40L.10
These data prompted us to institute a multicenter collaborative study of a larger series of ATR-associated PCs to determine specific factors that can be targeted generally or in certain donors or patients to further reduce proinflammatory sCD40L production during collection and storage of platelet components.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Garraud, EFS Auvergne-Loire and GIMAP-EA 3064, Université de Saint-Etienne, Faculté de Médecine, 15 rue Ambroise Paré, 42023 Saint-Etienne cedex 2, France; e-mail: olivier.garraud@efs.sante.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal