In this issue of Blood, Gelebart and colleagues show that the Wnt canonical pathway is constitutively active in mantle cell lymphoma cells and that selective inhibition of the pathway decreases malignant B-cell growth.
The Wnt signaling pathway is a highly conserved system that has a key role in embryonic development and in the growth and maintenance of normal tissues.1 It has also been shown to have important roles in lymphopoiesis and hematopoiesis.2 Dysregulated expression of components of the Wnt pathway can induce transforming events and are known to contribute to the pathogenesis of various malignancies.
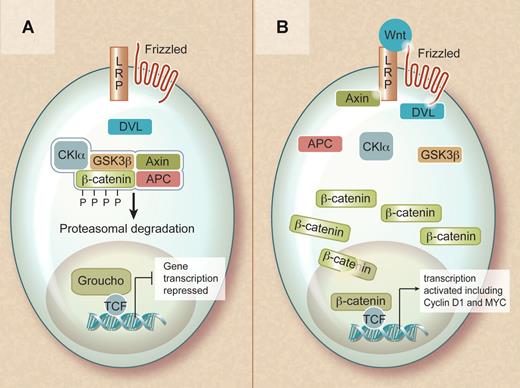
The Wnt family consists of secreted factors that bind to frizzled receptors (a family of transmembrane proteins) and low-density lipoprotein receptor-related protein coreceptors (LRP5 or LRP6) in the plasma membrane. In the absence of a Wnt ligand, β-catenin is found in a cytoplasmic complex with axin and adenomatous polyposis coli (APC) proteins and is targeted for ubiquitin-mediated degradation upon its phosphorylation by casein kinase 1α (CK1α) and glycogen synthase kinase 3β (GSK3β). Consequently, in the nonactivated state, cytoplasmic β-catenin levels remain low and lymphocyte enhancer binding factor (LEF) and T-cell factor (TCF) in the nucleus interact with Grouchos to repress Wnt-specific target genes.
Overview of the Wnt signaling pathway. (A) In the absence of a Wnt signal, β-catenin is captured within a destruction complex and phosphorylated. This results in ubiquitinylation and proteasomal degradation of β-catenin, ensuring repression of its target genes. (B) In the presence of a Wnt ligand, the destruction complex is inactivated and β-catenin translocates to the nucleus. In the nucleus, β-catenin becomes part of a transcriptionally active complex, ensuring efficient activation of its target genes. Professional illustration by Debra T. Dartez.
Overview of the Wnt signaling pathway. (A) In the absence of a Wnt signal, β-catenin is captured within a destruction complex and phosphorylated. This results in ubiquitinylation and proteasomal degradation of β-catenin, ensuring repression of its target genes. (B) In the presence of a Wnt ligand, the destruction complex is inactivated and β-catenin translocates to the nucleus. In the nucleus, β-catenin becomes part of a transcriptionally active complex, ensuring efficient activation of its target genes. Professional illustration by Debra T. Dartez.
In contrast, when a Wnt protein binds to the receptor complex, β-catenin is no longer phosphorylated and targeted for degradation, resulting in accumulation of β-catenin in the cytoplasm and its translocation to the nucleus. In the nucleus, β-catenin regulates gene expression in cooperation with members of the TCF and LEF family of transcription factors. This results in the activation of multiple target genes, including cyclin D1 and c-MYC, that instruct the cell to actively proliferate and remain in an undifferentiated state.
Mantle cell lymphoma is a well defined subtype of B-cell non-Hodgkin lymphoma and is characterized by dysregulated cyclin D1 gene expression.3 This dysregulation is secondary to the t(11;14)(q13;q32) translocation that juxtaposes the proto-oncogene CCND1 at chromosome 11q13, which encodes cyclin D1, to the immunoglobulin heavy chain gene at chromosome 14q32. Cyclin D1, which is not expressed in normal B cells, becomes constitutively overexpressed, resulting in deregulation of the cell cycle, alterations in DNA damage response pathways, and activation of cell survival mechanisms. However, overexpression of cyclin D1 alone is not sufficient for tumor formation, suggesting that dysregulation of additional signaling pathways may be critical for development of mantle cell lymphoma.4
Gelebart and colleagues have now shown that the Wnt canonical pathway is constitutively activated in a subset of patients with mantle cell lymphoma and that activation of the Wnt pathway appears to promote tumorigenesis. They found that Wnt3 and Wnt10 are highly expressed in mantle cell lymphoma and that β-catenin is localized to the nucleus and transcriptionally active in mantle cell lymphoma cell lines. Fifty percent of biopsy specimens from mantle cell lymphoma patients showed nuclear localization of β-catenin, and this correlated with an increase in the inactive form of GSK3β. The functional relevance of the Wnt canonical pathway in mantle cell lymphoma was further confirmed by selective inhibition of β-catenin resulting in decreased growth of the malignant B cells. These findings are supported by previous gene expression profiling studies that showed that several genes from the Wnt signaling pathway were up-regulated in mantle cell lymphoma cells.5
These results suggest that the Wnt pathway promotes malignant cell growth in mantle cell lymphoma and the Wnt pathway may therefore be a target for therapeutic intervention. Small molecule inhibitors and monoclonal antibodies targeting this pathway are being developed and may be beneficial in treating mantle cell lymphoma in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal