Abstract
Hematopoiesis during development is a dynamic process, with many factors involved in the emergence and regulation of hematopoietic stem cells (HSCs) and progenitor cells. Whereas previous studies have focused on developmental signaling and transcription factors in embryonic hematopoiesis, the role of well-known adult hematopoietic cytokines in the embryonic hematopoietic system has been largely unexplored. The cytokine interleukin-1 (IL-1), best known for its proinflammatory properties, has radioprotective effects on adult bone marrow HSCs, induces HSC mobilization, and increases HSC proliferation and/or differentiation. Here we examine IL-1 and its possible role in regulating hematopoiesis in the midgestation mouse embryo. We show that IL-1, IL-1 receptors (IL-1Rs), and signaling mediators are expressed in the aorta-gonad-mesonephros (AGM) region during the time when HSCs emerge in this site. IL-1 signaling is functional in the AGM, and the IL-1RI is expressed ventrally in the aortic subregion by some hematopoietic, endothelial, and mesenchymal cells. In vivo analyses of IL-1RI–deficient embryos show an increased myeloid differentiation, concomitant with a slight decrease in AGM HSC activity. Our results suggest that IL-1 is an important homeostatic regulator at the earliest time of HSC development, acting to limit the differentiation of some HSCs along the myeloid lineage.
Introduction
The cytokine interleukin-1 (IL-1) plays a role in a range of physiologic processes and is best known for its role as a major inflammatory mediator. IL-1 has been implicated as a regulator of bone marrow (BM) hematopoietic stem cells (HSCs) and progenitor cells,1-4 and these cells express IL-1 and its high-affinity receptor (IL-1 receptor type I [IL-1RI] and accessory chain).5-7 IL-1–mediated effects on HSCs and progenitor cells include radioprotection, changes in cell adhesion and migration, and modified cell growth and/or differentiation. When injected before high-dose irradiation, IL-1 enhances survival rates and maintains BM HSCs.8-10 The increased resistance to cytotoxicity is attributed to IL-1–induced cell cycle effects and up-regulation of the antioxidant enzyme manganese superoxidase dismutase (Sod2).11,12 IL-1 also promotes the mobilization of HSCs and affects BM endothelium to enhance the transendothelial migration of hematopoietic cells13,14 through factors, such as stromal cell–derived factor 1 (SDF1), CXC chemokine receptor 4 (CXCR4), and matrix metalloproteinase 9 (MMP9).15-18 In addition, the IL-1–induced up-regulated production of IL-6, granulocyte-macrophage colony-stimulating factor, and stem cell factor (SCF or kit ligand)2,9,19 is thought to accelerate hematopoietic recovery on irradiation through the expansion of hematopoietic progenitor and myeloid precursor cells.1,3,4 In mediating an inflammatory response, IL-1 stimulates the release of prostaglandins; recently, it has been shown that ex vivo exposure of adult mouse bone marrow to prostaglandin E2 enhances HSC activity.20 Some studies, however, report that IL-1 induces differentiation rather than expansion of hematopoietic progenitor cells or abrogates HSC activity.21,22 Depending on the target tissue, cellular context, and local concentration, IL-1 appears to have a broad range of biologic activities in the adult. However, nothing is known concerning a role for IL-1 in embryonic hematopoiesis
During mouse development, the first primitive hematopoietic cells are found in the yolk sac from embryonic day 7.5 (E7.5).23 Thereafter, many hematopoietic progenitors are found in the yolk sac, intraembryonic para-aortic splanchnopleura, and chorioallantoic placenta.24-26 At E10.5, the first adult repopulating HSCs are generated in the aorta-gonad-mesonephros (AGM) region and are localized in the endothelium/cell clusters of the ventral aspect of the dorsal aorta (also the vitelline and umbilical arteries).27,28 From E11 to E12 onwards, definitive HSCs are also detected in the yolk sac, placenta, circulation, and fetal liver (FL).29-32 The liver is the main fetal hematopoietic organ until birth, when the BM becomes the HSC niche.
The factors that play a role in the regulation of HSCs during development have been a focus of intense research interest. In our search for regulators of AGM HSCs, we reported that the novel gene Map3k7ip2 (mTAB2) is up-regulated between E10 and E11 in the mouse aorta in/near the endothelium.33 Human transforming growth factor-β–activated kinase 1 (TAK1)–binding protein 2 (TAB2) was originally identified as a binding partner of the mitogen-activated protein kinase (MAPK) family member TAK1 and is involved in IL-1 and tumor necrosis factor (TNF) signaling.34,35 TAB2 has been proposed to function as an adapter protein, binding TAK1 to TNF receptor–associated factor 6 (TRAF6) and bringing them to the IL-1 receptor complex, resulting in nuclear factor–kappa B (NF-κB) and c-Jun N-terminal kinase (JNK) activation.34,35 Because the expression pattern of mTAB2 in the dorsal aorta endothelium correlates with the emergence of HSC activity in this region, we set out to investigate whether IL-1 is a regulator of HSCs in the midgestation embryo.
Here, we show that IL-1 signaling is functional in the AGM region from E11 onwards. IL-1RI expression is localized to the ventral aspect of the dorsal aorta in hematopoietic, endothelial, and mesenchymal cells. The absence of IL-1RI results in an increase in myeloid progenitor and mature hematopoietic cell numbers and a slight decrease in HSC activity, suggesting that, in vivo, in the AGM region, IL-1RI–mediated signaling is negatively regulating the differentiation of HSCs along the myeloid lineage. Thus, IL-1, generally thought to be an adult cytokine, plays a role in the normal regulation of hematopoietic progenitor and stem cells in the midgestation mouse AGM.
Methods
Embryo generation
Animals were housed according to institutional guidelines, with free access to water and food. Procedures were carried out in compliance with the Standards for Humane Care and Use of Laboratory Animals. Matings for embryo generation were set up between (CBA × C57BL/10)F1 females and Ln72 human β-globin36 males, C57BL/6 females and Act-GFP males,37 C57BL/6 males and females, and Il1r1−/− (Il1rtm1Imx/tm1Imx)38 males and females. The day of the vaginal plug was counted as day 0. Pregnant mice were killed, embryos isolated, and AGMs and livers were dissected.39 Tissues were assayed directly or after 3-day explant culture. Single-cell suspensions were prepared after tissues were treated with collagenase (0.125% in phosphate-buffered saline [PBS]/10% fetal calf serum [FCS]/1% penicillin/streptomycin [Pen/Strep]) for 1 hour at 37°C.
Cell culture
3T3 fibroblasts were cultured in Dulbecco modified Eagle medium (DMEM)/10% FCS/1% Pen/Strep or overnight in DMEM/1% FCS/1% Pen/Strep. 3T3 cells were treated with 10 pg/mL to 100 ng/mL IL-1β (PeproTech, Rocky Hill, NJ) for the indicated times. Cell suspensions from several AGM (or liver) tissues were pooled, and 2 to 4 tissue equivalents were seeded in 6-well plates in DMEM/10%FCS/1% Pen/Strep. IL-1β was added the next day for target gene induction and IκB degradation studies.
UG26-1B6 cells40 were cultured at 33°C in long-term culture–stromal medium (LTC-SM) medium containing 50% M5300 (StemCell Technologies, Vancouver, BC)/15% FCS/35% alpha-minimum essential medium/1% Pen/Strep/10 μM β-mercaptoethanol and stimulated with 10 ng/mL IL-1β for 2, 6, and 24 hours.
Progenitor assay
After isolation of freshly dissected AGMs or 3-day AGM explant cultures with or without IL-1β, cell suspensions from pooled AGMs (3 or 4) were made; 0.5 × 104 to 7.5 × 104 cells per plate were seeded in methylcellulose medium (Methocult GF M3434; Stem Cell Technologies) containing SCF, IL-3, IL-6, and erythropoietin and incubated at 37°C, 5% CO2. Colony-forming unit–granulocyte, macrophage, granulocyte-macrophage, and granulocyte erythroid megakaryocyte macrophage (CFU-G, -M, -GM, and -GEMM, respectively) and burst-forming unit–erythroid (BFU-E) were scored with an inverted microscope at day 7 of culture.
Western blotting
E11-E12 AGM or liver cells or 3T3 cells were stimulated with 10 ng/mL and 100 ng/mL IL-1β, respectively, for 0 to 30 minutes. Protein lysates were made with radioimmunoprecipitation assay lysis buffer, separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted on polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were blocked with 4% nonfat milk (Bio-Rad, Hercules, CA)/TBS-T and incubated with an anti-IκB antibody (Cell Signaling Technology, Danvers, MA) or antitubulin antibodies followed by horseradish peroxidase–conjugated secondary antibodies (DAKO, Carpinteria, CA) and visualized with enhanced chemiluminescence detection.
Immunohistochemistry
Embryos were snap-frozen in TissueTek (Sakura, Zoeterwoude, The Netherlands) and 7- to 10-μM cryosections generated. Sections were fixed in 2% paraformaldehyde/PBS, and endogenous peroxidase activity was blocked with 1.2% H2O2/MeOH. Sections were incubated with anti–IL-1RI antibody (clone 12A6; BD Biosciences, San Jose, CA) overnight at 4°C. Sections were incubated with α-rat-biotin antibody and streptavidin–horseradish peroxidase (DAKO) in a tyramide signal amplification–biotin system (PerkinElmer, Waltham, MA). Staining was visualized with diaminobenzidine (DAB) chromogen (Sigma-Aldrich, St Louis, MO). Sections were counterstained with hematoxylin and embedded in Entallan (Merck, Darmstadt, Germany).
Explant cultures and in vivo transplantation assays
E11 AGMs (green fluorescent protein [GFP] or human β-globin transgene) were dissected, and 3-day explant cultures were performed28 in the presence of 0, 1, or 10 ng/mL IL-1β (PeproTech). Single-cell suspensions from pooled AGMs were obtained, and different cell dilutions (embryo equivalents) were coinjected intravenously with nonmarked spleen cells (2 × 105) into 9.0 Gy-irradiated (CBA × C57BL/10)F1 mice. Il1r1−/− and Act-GFP AGM cells were (directly or after explant culture) transplanted into (129SV × C57BL/6) adults. Repopulation was assayed at 1 and 4 months after transplantation by donor-specific semiquantitative polymerase chain reaction (PCR; human β-globin, GFP, or Neo) on peripheral blood DNA.27,28,36 Only mice with more than 10% donor chimerism were considered repopulated. For multilineage repopulation analysis, DNA was isolated from spleen, thymus, BM, lymph node, and peripheral blood or from fluorescence-activated cell sorter (FACS)–sorted cells from these tissues, and assayed for donor contribution by PCR.
FACS analysis
Single-cell suspensions were stained with anti–IL-1RI antibody (phycoerythrin [PE], clone 35F5) in PBS/10% FCS/1% Pen/Strep on ice for 20 to 30 minutes. Cells were costained with fluorescein isothiocyanate (FITC)–labeled antibodies (BD Biosciences) for c-kit (CD117), Mac1 (CD11b), CD45, or CD31. Other FACS analyses were performed with FITC–anti-Mac1 (CD11b), PE–anti-CD34 (RAM34), allophycocyanin–anti–c-kit (CD117) (clone 2B8), peridinin chlorophyll protein (PerCP) Cy5.5–anti-CD45 or CXCR4, followed by streptavidin-PE (Caltag Laboratories, Burlingame, CA) or anti–rat-PE and annexin V–FITC antibodies. Dead cells were excluded by 7-aminoactinomycin D (Molecular Probes, Eugene, OR) or Hoechst 33258 (1 μg/mL; Molecular Probes). Analysis was performed on FACScan or FACSAria (BD Biosciences) and with CellQuest software. For intracellular FACS, AGM single-cell suspensions were made in the presence of GolgiPlug (BD Biosciences). Cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences). Thereafter, cells were stained with a PE-conjugated anti–IL-1α antibody (clone ALF161; BD Biosciences) and analyzed on a FACScan (BD Biosciences).
RNA isolation, cDNA synthesis, and reverse transcriptase–PCR analysis
Total RNA was isolated with TRIzol (Invitrogen) and treated with RQ1 RNase-free DNAse (Promega, Madison, WI). For cDNA synthesis, 1 to 5 μg total RNA was reverse-transcribed with Superscript II (Invitrogen). PCRs were performed in 50 μL with 1 U Amplitaq (PerkinElmer), 100 ng of each primer, 1 mM dNTPs, and 0.5 to 2 μL cDNA. PCR cycles: 5 minutes 92°C, 27 to 40 times (40 seconds 92°C, 40 seconds 56/58/63°C, 1-2 minutes 72°C), 7 minutes 72°C. Primers are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). PCR products were run on 1.5% agarose/1× Tris–boric acid–EDTA buffer (TBE) gels, scanned (Typhoon; GE Healthcare, Little Chalfont, United Kingdom), and analyzed with ImageQuant software.
Results
IL-1–signaling components are expressed and function in the midgestation AGM
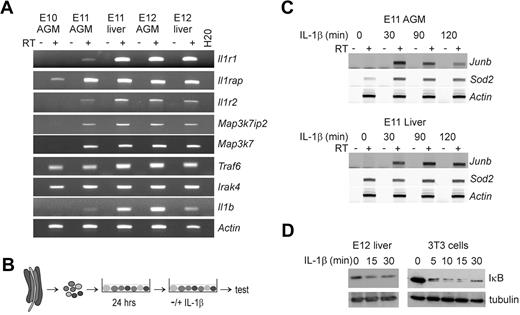
Cells from the midgestation AGM region were examined for expression of IL-1, its receptors, and signaling components. RT-PCR analysis (Figure 1A) shows the expression of IL-1Rs, Il1r1 (receptor type I) and Il1rap (receptor-associating protein) in the E11 and E12 AGM and FL. Il1rap expression is already initiated at E10. The nonsignaling Il1r2 (receptor type II) is detected from E11 onwards in the AGM and FL. Intracellular-signaling components, Map3k7ip2 (TAB2) and Map3k7 (TAK1), are also expressed in the AGM and FL at E11 and onwards. Other essential IL-1R–signaling components, Traf6 and Irak4, are expressed earlier, from E10 onwards, in both AGM and FL. Moreover, Il1β is expressed in the AGM region beginning at E11 and increased at E12. Thus, all the essential IL-1–signaling components are expressed in the E11-E12 AGM and FL.
IL-1R–signaling components are expressed and functional in the midgestation AGM region and liver. (A) RT-PCR analysis performed to examine the expression of the IL-1Rs type I (Il1r1), the accessory receptor (Il1rap) and the receptor type II (Il1r2), and several downstream signaling components, including Map3k7ip2 (TAB2), Map3k7 (TAK1), Traf6, and Irak4, and the ligand IL-1β (Il1b) in the E10-E12 AGM region and E11-E12 FL. (B) Overview of the culture method used to study gene induction or IκB degradation in AGM tissues. Single-cell suspensions were made from E11 AGM tissues and cultured in 6-well plates overnight. The next day, cells were treated with IL-1β and harvested for RT-PCR analysis or IκB degradation studies. (C) Representative semiquantitative RT-PCR for the IL-1β target genes Junb and Sod2 (MnSOD) after stimulation of E11 AGM and liver single-cell suspensions with IL-1β (10 ng/mL) for 0, 30, 90, or 120 minutes. (D) Western blot showing rapid IκB degradation after IL-1β stimulation (10 ng/mL) of E12 liver cells (left panel) or 3T3 fibroblasts (right panel).
IL-1R–signaling components are expressed and functional in the midgestation AGM region and liver. (A) RT-PCR analysis performed to examine the expression of the IL-1Rs type I (Il1r1), the accessory receptor (Il1rap) and the receptor type II (Il1r2), and several downstream signaling components, including Map3k7ip2 (TAB2), Map3k7 (TAK1), Traf6, and Irak4, and the ligand IL-1β (Il1b) in the E10-E12 AGM region and E11-E12 FL. (B) Overview of the culture method used to study gene induction or IκB degradation in AGM tissues. Single-cell suspensions were made from E11 AGM tissues and cultured in 6-well plates overnight. The next day, cells were treated with IL-1β and harvested for RT-PCR analysis or IκB degradation studies. (C) Representative semiquantitative RT-PCR for the IL-1β target genes Junb and Sod2 (MnSOD) after stimulation of E11 AGM and liver single-cell suspensions with IL-1β (10 ng/mL) for 0, 30, 90, or 120 minutes. (D) Western blot showing rapid IκB degradation after IL-1β stimulation (10 ng/mL) of E12 liver cells (left panel) or 3T3 fibroblasts (right panel).
The functional responsiveness of AGM and FL cells to IL-1 was measured by target gene induction (Junb and Sod2) and NF-κB pathway activation (IκB degradation). The conditions for the transcriptional induction of Junb by IL-1β were first determined in embryonic 3T3 fibroblasts. As determined by RT-PCR, 10 ng/mL IL-1β yielded a 3.9-fold increase in Junb expression after 2 hours of IL-1β stimulation (Figure S1A,B). When stimulated in the presence of an IL-1β–neutralizing antibody, Junb levels were reduced to those observed in untreated 3T3 cells (Figure S1C), thus demonstrating the specificity of the assay. Cell suspensions of E10, E11, and E12 AGM and FL tissues were prepared and cells were cultured overnight before IL-1β stimulation (Figure 1B). When treated for 30 to 120 minutes with IL-1β, Junb expression rapidly increased in E11 and E12 AGM and FL cells (range, 1.7- to 60-fold) compared with untreated cells (Figure 1C; and data not shown). Consistent with the observation that Il1r1 is not expressed by E10 AGM cells, we did not detect IL-1β–induced Junb expression in E10 AGM cells (data not shown). IL-1β also induced the up-regulation of Sod2 gene in AGM cells (2.5- to 8-fold). IL-1β–mediated effects on the NF-κB pathway were also tested in this culture system by Western blot analysis for IκB. After IL-1β stimulation, IκB degradation in both E12 FL and 3T3 cells was observed (Figure 1D), along with a moderate reduction of IκB protein in E11/E12 AGM and E11 FL (data not shown). Thus, populations of cells within the E11/ E12 AGM and FL are IL-1– responsive and display functional IL-1–signaling properties.
IL-1R expression is localized to the midgestation dorsal aorta
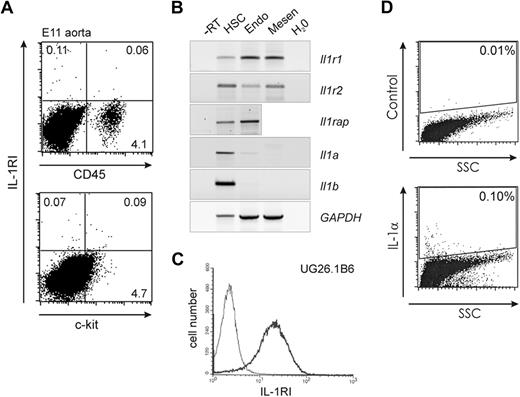
To determine in which AGM cell lineage IL-1RI is expressed, immunohistochemistry, flow cytometric and RT-PCR analyses were performed. Immunostainings on E11 transverse sections localize IL-1RI protein expression to the cells along the ventral wall of the aorta and cells directly underlying the wall (presumably endothelial and mesenchymal cells, respectively; Figure 2B). A few circulating cells and cells scattered through the mesenchymal areas of the AGM also appear faintly positive (Figure 2C), with the localization suggesting that these may be hematopoietic cells and tissue macrophages. At E11, expression is low and limited to the cells on the ventral aspect of the aorta, whereas at E12 IL-1RI expression is found in the same cell types but spread around the entire aorta (not shown).
IL-1RI is expressed by cells in the E11 region aortic hematopoietic, endothelial, and mesenchymal cells. Immunostaining performed with (A) a control antibody and (B,C) an IL-1RI–specific antibody on transverse cryosections from E11 embryos. (C) An enlargement of the boxed area in panel B. The dorsal aorta of the AGM region is shown with the ventral side at the bottom of the section. Positive signal is seen as an orange-brown precipitate from the DAB chromogen. The sections were counterstained with hematoxylin. Orange arrowheads indicate single IL-1RI–expressing cells in the circulation and scattered in the tissue. Slides were viewed with an Olympus BX40 research microscope (Olympus Nederland B.V., Zoetewoude, The Netherlands) using an Olympus lens at 20×/0.40 PH. Images were acquired and processed with Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA).
IL-1RI is expressed by cells in the E11 region aortic hematopoietic, endothelial, and mesenchymal cells. Immunostaining performed with (A) a control antibody and (B,C) an IL-1RI–specific antibody on transverse cryosections from E11 embryos. (C) An enlargement of the boxed area in panel B. The dorsal aorta of the AGM region is shown with the ventral side at the bottom of the section. Positive signal is seen as an orange-brown precipitate from the DAB chromogen. The sections were counterstained with hematoxylin. Orange arrowheads indicate single IL-1RI–expressing cells in the circulation and scattered in the tissue. Slides were viewed with an Olympus BX40 research microscope (Olympus Nederland B.V., Zoetewoude, The Netherlands) using an Olympus lens at 20×/0.40 PH. Images were acquired and processed with Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA).
Flow cytometric analyses revealed that, on average, 0.16% of E11 and 0.33% of E12 aorta cells express IL-1RI (Table 1). On average, 45% of E11 IL-1RI–positive cells express CD45 or c-kit (Table 1; Figure 3A). Thus, some IL-1RI–expressing cells are of the hematopoietic lineage or possibly endothelial cells taking on hematopoietic fate. To confirm the expression of IL-1RI in the different lineages, E11 aorta cells were sorted based on hematopoietic (c-kit+CD34+), endothelial (VE-cadherin+CD45−), or presumed mesenchymal (vascular endothelial [VE]–cadherin−CD45−) marker phenotypes and examined by RT-PCR. Cells of all 3 lineages express the Il1r1 and Il1r2 genes but to various degrees (Figure 3B). HSCs appear to express less Il1r1 and more Il1r2 than endothelial or presumed mesenchymal cells. HSCs and endothelial cells also express Il1rap (VE-cadherin−CD45− cells not tested). The lack of a positive selection method for mesenchymal cells from the AGM prompted us to examine several well-characterized E11 AGM-derived mesenchymal cell lines for expression of Il1r1 and Il1rap. RT-PCR revealed Il1r1 and Il1rap expression in all tested embryonic stromal cell lines (Table 2). Flow cytometric analysis shows a high expression of IL-1RI on one of the best hematopoietic supportive AGM stromal lines, UG26-1B6 (Figure 3C). Whereas IL-1Rs are expressed by the 3 populations of cells, the ligand genes Il1a and Il1b are expressed primarily by HSCs. Intracellular flow cytometric analysis verified the expression of IL-1α protein by AGM cells, showing 0.10% of AGM cells positive (Figure 3D). Thus, the expression of IL-1RI on some AGM hematopoietic, endothelial, and mesenchymal stromal cells suggests a possible role for IL-1 signaling in this tissue.
Percentages of IL-1RI-, CD45-, and c-kit–expressing aorta cells
| . | E11 aorta . | E12 aorta . | ||
|---|---|---|---|---|
| Mean . | Range . | Mean . | Range . | |
| IL-1RI+/CD45− | 0.09 | 0.05-0.11 | 0.22 | 0.12-0.43 |
| IL-1RI+/CD45+ | 0.07 | 0.05-0.11 | 0.11 | 0.01-0.16 |
| IL-1RI−/CD45+ | 3.99 | 3.83-4.08 | 3.25 | 2.81-3.98 |
| IL-1RI+/c-kit− | 0.09 | 0.07-0.12 | 0.16 | 0.14-0.19 |
| IL-1RI+/c-kit+ | 0.06 | 0.03-0.09 | 0.15 | 0.03-0.22 |
| IL-1RI−/c-kit+ | 6.60 | 4.86-9.83 | 8.35 | 5.31-13.05 |
| . | E11 aorta . | E12 aorta . | ||
|---|---|---|---|---|
| Mean . | Range . | Mean . | Range . | |
| IL-1RI+/CD45− | 0.09 | 0.05-0.11 | 0.22 | 0.12-0.43 |
| IL-1RI+/CD45+ | 0.07 | 0.05-0.11 | 0.11 | 0.01-0.16 |
| IL-1RI−/CD45+ | 3.99 | 3.83-4.08 | 3.25 | 2.81-3.98 |
| IL-1RI+/c-kit− | 0.09 | 0.07-0.12 | 0.16 | 0.14-0.19 |
| IL-1RI+/c-kit+ | 0.06 | 0.03-0.09 | 0.15 | 0.03-0.22 |
| IL-1RI−/c-kit+ | 6.60 | 4.86-9.83 | 8.35 | 5.31-13.05 |
For E11, n = 3 and for E12, n = 4.
IL-1RI is expressed by cells in the E11 region aortic hematopoietic, endothelial, and mesenchymal cells. (A) Representative flow cytometric dot plots showing E11 aorta cells (n = 4) stained with antibodies specific for IL-1RI and CD45 (top panel) or c-kit (bottom panel). Percentages of cells in each quadrant are indicated; 3 to 3.6 × 104 events are shown, and more than 5 × 104 events were analyzed. (B) RT-PCR analysis for IL-1R and ligand expression in sorted HSCs (CD34+c-kit+), endothelial cells (CD45−VE-cadherin+; Endo), and presumed mesenchymal cells (CD45−VE-cadherin−; Mesen) from the E11 aorta. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression serves as the normalization control. −RT indicates no reverse transcriptase control. (C) Flow cytometric analysis of IL-1RI expression on UG26-1B6 stromal cells. X-axis indicates intensity of fluorescent signal from staining with IL-1RI–specific antibody; y-axis indicates the number of cells. (D) Intracellular flow cytometric dot plots showing control antibody (top panel) and anti–IL-1α antibody (bottom panel) staining in E11 AGM cells. Boxed areas indicate percentage positive cells.
IL-1RI is expressed by cells in the E11 region aortic hematopoietic, endothelial, and mesenchymal cells. (A) Representative flow cytometric dot plots showing E11 aorta cells (n = 4) stained with antibodies specific for IL-1RI and CD45 (top panel) or c-kit (bottom panel). Percentages of cells in each quadrant are indicated; 3 to 3.6 × 104 events are shown, and more than 5 × 104 events were analyzed. (B) RT-PCR analysis for IL-1R and ligand expression in sorted HSCs (CD34+c-kit+), endothelial cells (CD45−VE-cadherin+; Endo), and presumed mesenchymal cells (CD45−VE-cadherin−; Mesen) from the E11 aorta. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression serves as the normalization control. −RT indicates no reverse transcriptase control. (C) Flow cytometric analysis of IL-1RI expression on UG26-1B6 stromal cells. X-axis indicates intensity of fluorescent signal from staining with IL-1RI–specific antibody; y-axis indicates the number of cells. (D) Intracellular flow cytometric dot plots showing control antibody (top panel) and anti–IL-1α antibody (bottom panel) staining in E11 AGM cells. Boxed areas indicate percentage positive cells.
Expression of IL-1–signaling molecules by embryonic stromal cell lines
| Cell line . | Origin . | Expression . | |||||
|---|---|---|---|---|---|---|---|
| IL-1RI(Il1r1) . | IL-1RacP(Il1rap) . | IL-1RII(Il1r2) . | IL-1a(Il1a) . | IL-1b(Il1b) . | Actin(Actb) . | ||
| AM20-1A4 | Ao | ++ | ++ | ++ | − | − | ++ |
| AM30-2A4 | Ao | ++ | ++ | ± | − | − | ++ |
| AM30-3F4 | Ao | ++ | ++ | − | − | − | ++ |
| AM30-3A3 | Ao | ++ | ++ | + | − | − | + |
| UG26-1B6 | UGR | ++ | ++ | − | − | − | + |
| EL08-1D2 | FL | ++ | ++ | − | − | − | + |
| EL23-1C2 | FL | ++ | ++ | − | − | − | + |
| FBMD-1 | BM | ++ | ++ | − | + | − | ++ |
| Cell line . | Origin . | Expression . | |||||
|---|---|---|---|---|---|---|---|
| IL-1RI(Il1r1) . | IL-1RacP(Il1rap) . | IL-1RII(Il1r2) . | IL-1a(Il1a) . | IL-1b(Il1b) . | Actin(Actb) . | ||
| AM20-1A4 | Ao | ++ | ++ | ++ | − | − | ++ |
| AM30-2A4 | Ao | ++ | ++ | ± | − | − | ++ |
| AM30-3F4 | Ao | ++ | ++ | − | − | − | ++ |
| AM30-3A3 | Ao | ++ | ++ | + | − | − | + |
| UG26-1B6 | UGR | ++ | ++ | − | − | − | + |
| EL08-1D2 | FL | ++ | ++ | − | − | − | + |
| EL23-1C2 | FL | ++ | ++ | − | − | − | + |
| FBMD-1 | BM | ++ | ++ | − | + | − | ++ |
IL-1R (IL-1RI and IL-1RAcP) expression is detected RT-PCR in several stromal cell lines derived from different embryonic and adult mouse tissues. No IL-1β expression could be detected in these cell lines and IL-1α expression was only detected in the adult BM-derived FBMD-1 stromal cell line. Expression of the IL-1 decoy receptor (IL-1RII) was detected in some of the stromal cell lines tested. Expression levels of these transcripts are indicated by their relative expression levels, with ++ indicating very high expression; +, high expression; ±, low expression; and −, no expression detected.
BM indicates bone marrow; Ao, dorsal aorta from AGM region; UGR, urogenital ridge from AGM region; and FL, fetal liver.
Exogenously added IL-1 affects mature hematopoietic cells, progenitors, and stem cells in the E11 AGM
The functional effect of IL-1 signaling on AGM hematopoiesis was first examined on E11 AGM explants cultured in different doses of IL-1β. Flow cytometric analysis for hematopoietic markers shows a dose-dependent IL-1β–induced increase in the absolute numbers of c-kit+, CD45+, and Mac1+ cells (Figure 4A). The number of c-kit+ cells increased on average 1.6- and 1.4-fold and CD45+ and Mac1+ cells both increased 1.6- and 2.3-fold when cultured in the presence of 1 ng/mL and 10 ng/mL IL-1β, respectively. Similar increases in the number of c-kit+ cells were found in E11 aorta subregions cultured in the presence of IL-1β (not shown).
IL-1β increases hematopoietic cells in the E11 AGM region. E11 AGM tissues were cultured for 3 days in the presence or absence of different doses of IL-1β. (A) Flow cytometric analysis showing the absolute number of cells per AGM positive for CD45, c-kit, or Mac1 after AGM explant culture (n = 5). (B) Number of CFU-G, CFU-M, and CFU-GEMM per E11 AGM explant cultured for 3 days in the presence of 0, 1, or 10 ng/mL IL-1β. Colonies were scored in triplicate cultures after 7 days of methylcellulose culture (n = 2). (C) Percentage of adult recipient mice repopulated with donor E11 AGM cells. E11 AGM explants were cultured in 0, 1, or 10 ng/mL IL-1β for 3 days, and cells were injected into irradiated recipients (1 embryo equivalent [ee]). At 4 months after transplantation, recipient peripheral blood DNA was analyzed for donor hematopoietic chimerism by semiquantitative PCR. Only mice with more than 10% donor chimerism were considered repopulated. Each column represents the number of mice repopulated per number of recipients transplanted (13 of 24, 9 of 13, and 4 of 12) with AGM explant cells cultured in 0, 1, and 10 ng/mL IL-1β, respectively). Combined results of 8 separate transplantation experiments. The error bars represent SEM.
IL-1β increases hematopoietic cells in the E11 AGM region. E11 AGM tissues were cultured for 3 days in the presence or absence of different doses of IL-1β. (A) Flow cytometric analysis showing the absolute number of cells per AGM positive for CD45, c-kit, or Mac1 after AGM explant culture (n = 5). (B) Number of CFU-G, CFU-M, and CFU-GEMM per E11 AGM explant cultured for 3 days in the presence of 0, 1, or 10 ng/mL IL-1β. Colonies were scored in triplicate cultures after 7 days of methylcellulose culture (n = 2). (C) Percentage of adult recipient mice repopulated with donor E11 AGM cells. E11 AGM explants were cultured in 0, 1, or 10 ng/mL IL-1β for 3 days, and cells were injected into irradiated recipients (1 embryo equivalent [ee]). At 4 months after transplantation, recipient peripheral blood DNA was analyzed for donor hematopoietic chimerism by semiquantitative PCR. Only mice with more than 10% donor chimerism were considered repopulated. Each column represents the number of mice repopulated per number of recipients transplanted (13 of 24, 9 of 13, and 4 of 12) with AGM explant cells cultured in 0, 1, and 10 ng/mL IL-1β, respectively). Combined results of 8 separate transplantation experiments. The error bars represent SEM.
Hematopoietic progenitor assays showed a 1.3-fold increase in the total number of CFU-Cs per AGM in cultures containing IL-1β (1 ng/mL) compared with the control cultures (not shown). Further analysis was focused on committed granulocyte (CFU-G) and macrophage (CFU-M) progenitors and multipotent granulocyte-erythroid-macrophage-megakaryocyte progenitors (CFU-GEMM) (Figure 4B) because IL-1 in the adult is known to promote myelopoiesis. As expected, the number of CFU-G per AGM and CFU-M per AGM was increased at both doses of IL-1β (Figure 4B). Interestingly, whereas there appeared to be a small increase in CFU-GEMM in AGM explants treated with 1 ng/mL IL-1β (2.5- to 4-fold), AGMs treated with high-dose IL-1β were significantly decreased in these immature progenitors. Thus, IL-1β expands the number of mature myeloid progenitors in AGM explants, whereas at a high dose it decreases the more immature progenitors.
Functional in vivo repopulation (HSC) assays further tested the effects of exogenously added IL-1β on immature hematopoietic progenitors. Cells from E11 AGM explants cultured in the presence of IL-1β (1 or 10 ng/mL) were transplanted into irradiated adult recipients. At 4 months after injection, recipient peripheral blood was analyzed for donor cell chimerism (Figure 4C). AGM explants cultured in low-dose IL-1β (1 ng/mL) showed a slight increase in HSC activity (69% of recipients repopulated) compared with control AGM cultures (54% of recipients repopulated). In contrast, HSC activity was decreased in the explants cultured in high-dose IL-1β (10 ng/mL), bringing HSC activity to levels lower than the control. These results are consistent with the dosage effects of IL-1β on CFU-GEMM and suggest that high levels of IL-1β disrupt both the AGM immature progenitor and HSC activity.
IL-1RI deficiency affects hematopoiesis in the E11 AGM
To determine whether IL-1β signaling is relevant in AGM hematopoiesis in a more physiologic setting, E11 IL-1RI–deficient tissues were examined. Phenotypic analysis showed 2.1-, 1.8-, and 1.5-fold increases in the absolute number, respectively, of c-kit+, CD45+, and Mac1+ hematopoietic cells in Il1r1−/− AGMs compared with Il1r1+/+ AGMs (Figure 5A). The absolute number of ckit+CD34+ cells (HSC enriched) was slightly increased (1.2-fold) in Il1r1−/− AGMs. After explant culture, Il1r1−/− AGMs contained similar numbers of c-kit+, CD45+, and Mac1+ hematopoietic cells (Figure 5B) in Il1r1−/− AGMs compared with in Il1r1+/+ AGMs.
IL-1R signaling affects AGM HSCs. (A) Flow cytometric analysis of freshly isolated E11 Il1r1+/+ and Il1r1−/− AGM cells showing the absolute number of c-kit+, CD45+, CD34+c-kit+, or CD34−Mac1+ cells per AGM (n = 3). (B) Flow cytometric analysis of E11 Il1r1+/+ and Il1r1−/− AGM explants showing absolute numbers of c-kit+, CD45+, or Mac1+ cells per AGM explant (n = 4-5). (C) Number of CFU total, CFU-M, BFU-E, CFU-G, CFU-GM, and CFU-GEMM per freshly isolated E11 Il1r1+/+ and Il1r1−/− AGM. Colonies from triplicate cultures were scored after 7 days of methylcellulose culture (n = 3, cells from 14 Il1r1+/+ and 15 Il1r1−/− AGMs). (D) Number of CFU total, CFU-M, BFU-E, CFU-G, CFU-GM, and CFU-GEMM per E11 Il1r1+/+ and Il1r1−/− AGM after 3 days of explant culture. Colonies from triplicate cultures were scored after 7 days of methylcellulose culture (n = 3, cells from 8 Il1r1+/+ and 8 Il1r1−/− AGMs). (E) Percentage of adult recipient mice repopulated with HSCs from Il1r1+/+ and Il1r1−/− AGM regions either directly transplanted (direct) or transplanted after 3 days of explant culture (explant). E11 AGM cells (1 and 0.3 or 1 and 0.2 embryo AGM tissue equivalents [ee]) were transplanted into irradiated adult recipients. At 4 months after transplantation, peripheral blood DNA of recipients was analyzed for donor hematopoietic chimerism by semiquantitative PCR. Only mice with more than 10% donor chimerism were considered repopulated. Each column represents the number of mice repopulated per number of recipients transplanted (the numbers for the Il1r1+/+ and Il1r1−/− columns are, respectively, 5 of 7 and 3 of 8 for 1 ee direct transplantation, 1 of 6 and 0 of 8 for 0.3 ee direct transplantation, 2 of 2 and 5 of 5 for 1 ee explant transplantation, and 8 of 10 and 2 of 10 for 0.2 ee explant transplantation). Combined results of 4 separate transplantation experiments. The error bars represent SEM.
IL-1R signaling affects AGM HSCs. (A) Flow cytometric analysis of freshly isolated E11 Il1r1+/+ and Il1r1−/− AGM cells showing the absolute number of c-kit+, CD45+, CD34+c-kit+, or CD34−Mac1+ cells per AGM (n = 3). (B) Flow cytometric analysis of E11 Il1r1+/+ and Il1r1−/− AGM explants showing absolute numbers of c-kit+, CD45+, or Mac1+ cells per AGM explant (n = 4-5). (C) Number of CFU total, CFU-M, BFU-E, CFU-G, CFU-GM, and CFU-GEMM per freshly isolated E11 Il1r1+/+ and Il1r1−/− AGM. Colonies from triplicate cultures were scored after 7 days of methylcellulose culture (n = 3, cells from 14 Il1r1+/+ and 15 Il1r1−/− AGMs). (D) Number of CFU total, CFU-M, BFU-E, CFU-G, CFU-GM, and CFU-GEMM per E11 Il1r1+/+ and Il1r1−/− AGM after 3 days of explant culture. Colonies from triplicate cultures were scored after 7 days of methylcellulose culture (n = 3, cells from 8 Il1r1+/+ and 8 Il1r1−/− AGMs). (E) Percentage of adult recipient mice repopulated with HSCs from Il1r1+/+ and Il1r1−/− AGM regions either directly transplanted (direct) or transplanted after 3 days of explant culture (explant). E11 AGM cells (1 and 0.3 or 1 and 0.2 embryo AGM tissue equivalents [ee]) were transplanted into irradiated adult recipients. At 4 months after transplantation, peripheral blood DNA of recipients was analyzed for donor hematopoietic chimerism by semiquantitative PCR. Only mice with more than 10% donor chimerism were considered repopulated. Each column represents the number of mice repopulated per number of recipients transplanted (the numbers for the Il1r1+/+ and Il1r1−/− columns are, respectively, 5 of 7 and 3 of 8 for 1 ee direct transplantation, 1 of 6 and 0 of 8 for 0.3 ee direct transplantation, 2 of 2 and 5 of 5 for 1 ee explant transplantation, and 8 of 10 and 2 of 10 for 0.2 ee explant transplantation). Combined results of 4 separate transplantation experiments. The error bars represent SEM.
CFU-C assays performed to evaluate the hematopoietic progenitor content and function showed a significant 1.7- to 2-fold increase in the number of CFU-G and CFU-M in Il1r1−/− AGMs compared with Il1r1+/+ AGMs (Figure 5C). However, no clear difference in number of BFU-E, CFU-GM, and CFU-GEMM was found. These data show that, in the absence of IL-1 signaling through IL-1RI, myeloid progenitors are expanded, suggesting that the balance between progenitor maintenance and hematopoietic differentiation is disrupted.
Surprisingly, when Il1r1−/− AGM explants were examined for CFU-Cs (Figure 5D), all clonogenic progenitors were decreased in number compared with Il1r1+/+ AGM explants. Il1r1−/− CFU-Cs did increase during the explant culture period compared with directly isolated AGMs. However, Il1r1−/− CFU-Cs were only 2-fold increased compared with the normal 6-fold increase found in the total numbers of Il1r1+/+ CFU-Cs (compare Figure 5C with 5D). Thus, IL-1RI–mediated signaling in vivo appears to limit the expansion of hematopoietic progenitor numbers (particularly myeloid progenitors), whereas in vitro, in the AGM explant culture, it is one of the necessary signals for hematopoietic progenitor (all CFC types) amplification.
Analysis of Il1r1−/− AGMs for HSC activity was performed by in vivo transplantation (direct or after explant culture). Direct transplantation will reveal the number of AGM HSCs in vivo, whereas transplantation after explant culture should reveal whether IL-1RI signaling affects HSC expansion in vitro. We found that directly transplanted Il1r1−/− AGMs were slightly decreased in HSC activity compared with wild-type AGMs (Figure 5E). After 3 days of culture, HSC activity in the Il1r1−/− AGM explants was less expanded compared with Il1r1+/+ explants. HSC-derived donor chimerism in the peripheral blood of recipients of Il1r1−/− E11 AGM cells was confirmed by multilineage analysis and secondary transplantations. High donor cell chimerism (32%-100%) was found in all hematopoietic tissues and lineages tested (Figure S2A). Moreover, high-level repopulation of secondary recipients revealed that the Il1r1−/− E11 AGM HSCs self-renew (Figure S2B). Thus, lack of signaling through IL-1RI–mediated signaling in the AGM affects HSC-repopulating activity but not potential of AGM HSCs and suggests a role for IL-1 in the normal physiologic growth of these cells in the embryo and their amplification in AGM explants in vitro.
IL-1 affects other regulators of hematopoietic cells
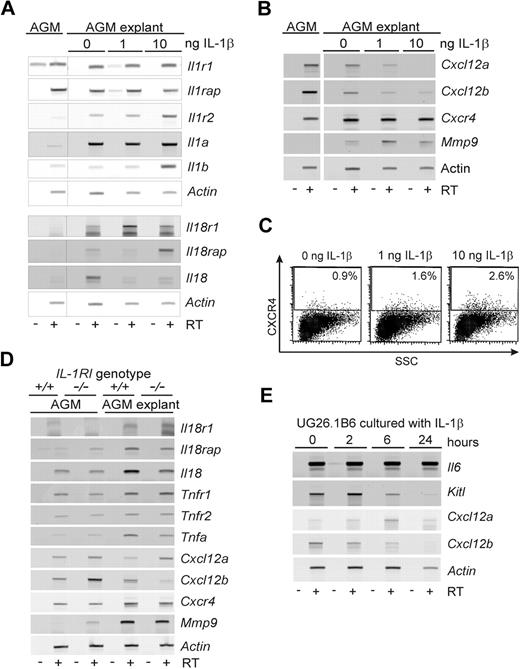
Semiquantitative RT-PCR analysis performed on E11 AGMs to examine whether IL-1β mediates changes in the expression of IL-1Rs, ligands, redundant signaling molecules (IL-18), and/or other downstream effectors, such as mobilization factors. Increases in Il1r2, Il1a, Il18r1, Il18rap, and Il18 expression (Figure 6A) were found in AGM explants compared with uncultured AGMs. Culture with exogenously added IL-1β (1 or 10 ng/mL) increased Il1r2, Il1b, and Il18rap expression in AGM explants, particularly at 10 ng/mL. At 1 ng/mL, IL-1β induced higher levels of Il18r1 but decreased Il18 expression. Thus, the IL-1β–induced increase in IL-1 production, together with an up-regulation of molecules in the IL-18–signaling pathway may in part influence the function of hematopoietic cells in the AGM explants.
Expression of IL-1–signaling molecules and hematopoietic regulators in IL-1β–stimulated AGMs. (A) RT-PCR analysis of E11 AGM tissue before and after 3-day explant culture in the presence of 0, 1, or 10 ng/mL IL-1β. Changes in gene expression of some of the tested genes (Il1r1, Il1rap, Il1r2, Il1a, Il1b, Il18r1, Il18rap, Il18) are found after explant culture or are induced by the presence of IL-1β. (B) RT-PCR analysis of E11 AGM tissue before and after 3-day explant culture in the presence of 0, 1, or 10 ng/mL IL-1β. Changes in gene expression of some of the tested genes (Cxcl12a and Cxcl12b, Cxcr4, Mmp9) are found after explant culture or are induced by the presence of IL-1β. (C) Flow cytometric analysis of cultured E11 aorta explants for expression of CXCR4. E11 aorta explants were cultured in the presence of 0, 1, or 10 ng/mL IL-1β for 3 days before analysis. The percentage of CXCR4+ cells is indicated in the gated upper section (n = 3); 3 × 104 events were analyzed, and 1.3 to 1.5 × 104 events are shown. (D) RT-PCR analysis of AGM tissue from Il1r1+/+ and Il1r1−/− E11 embryos. AGM tissues before and after 3-day explant culture were used for RNA preparation. Changes in gene expression of some of the tested genes (Il81r1, Il81rap, Il18, Tnfr1, Tnfr2, Tnfa, Cxcl12a, Cxcl12b, Cxcr4, Mmp9) are found in the absence of IL-1RI directly or after explant culture; n = 2-3 for each gene. (E) RT-PCR analysis of UG26-1B6 cells treated with 10 ng/mL IL-1β for various times (2-24 hours) and examined for gene expression of several hematopoietic cytokines (Il6, Kitl) and chemokines (Cxcl12a, Cxcl2b). Representative experiments are shown in panels A, B, C, and E (n = 2). Actin was used as a cDNA normalization control. −RT indicates no reverse transcriptase; +RT, + reverse transcriptase.
Expression of IL-1–signaling molecules and hematopoietic regulators in IL-1β–stimulated AGMs. (A) RT-PCR analysis of E11 AGM tissue before and after 3-day explant culture in the presence of 0, 1, or 10 ng/mL IL-1β. Changes in gene expression of some of the tested genes (Il1r1, Il1rap, Il1r2, Il1a, Il1b, Il18r1, Il18rap, Il18) are found after explant culture or are induced by the presence of IL-1β. (B) RT-PCR analysis of E11 AGM tissue before and after 3-day explant culture in the presence of 0, 1, or 10 ng/mL IL-1β. Changes in gene expression of some of the tested genes (Cxcl12a and Cxcl12b, Cxcr4, Mmp9) are found after explant culture or are induced by the presence of IL-1β. (C) Flow cytometric analysis of cultured E11 aorta explants for expression of CXCR4. E11 aorta explants were cultured in the presence of 0, 1, or 10 ng/mL IL-1β for 3 days before analysis. The percentage of CXCR4+ cells is indicated in the gated upper section (n = 3); 3 × 104 events were analyzed, and 1.3 to 1.5 × 104 events are shown. (D) RT-PCR analysis of AGM tissue from Il1r1+/+ and Il1r1−/− E11 embryos. AGM tissues before and after 3-day explant culture were used for RNA preparation. Changes in gene expression of some of the tested genes (Il81r1, Il81rap, Il18, Tnfr1, Tnfr2, Tnfa, Cxcl12a, Cxcl12b, Cxcr4, Mmp9) are found in the absence of IL-1RI directly or after explant culture; n = 2-3 for each gene. (E) RT-PCR analysis of UG26-1B6 cells treated with 10 ng/mL IL-1β for various times (2-24 hours) and examined for gene expression of several hematopoietic cytokines (Il6, Kitl) and chemokines (Cxcl12a, Cxcl2b). Representative experiments are shown in panels A, B, C, and E (n = 2). Actin was used as a cDNA normalization control. −RT indicates no reverse transcriptase; +RT, + reverse transcriptase.
Analyses of HSC mobilization factors (Figure 6B) showed an IL-1β–mediated dose-dependent decrease in Cxcl12a and Cxcl12b expression (SDF1-α and -β isoforms) in AGM explants. Cxcr4 expression was slightly increased in the AGM explants and was independent of exogenously added IL-1β. Mmp9 expression was induced in AGM explants, with some further induction occurring with 1 ng/mL IL-1β. FACS analysis performed on aorta explants cultured in the presence or absence of IL-1β showed a dose-dependent increase in the percentage of CXCR4+ cells (Figure 6C).
As these signaling modulators may play a role in compensating for the loss of IL-1RI-mediated signaling in Il1r1−/− E11 AGMs, we performed RT-PCR for the expression of some of these hematopoietic modulators before and after AGM explant culture (Figure 6D). No major changes were observed in the expression of Il18 and Tnf-related molecules compared with Il1r1+/+ AGMs. Only the expression of Cxcl12b was increased in Il1r1−/− AGMs before culture and decreased after 3 days of culture compared with Il1r1+/+ AGMs. In addition, a small increase in Mmp9 expression was observed in Il1r1−/− AGMs before culture.
As a more direct means of examining the AGM microenvironment, UG26-1B6 stromal cells were stimulated for 2 to 24 hours with IL-1β. RT-PCR for several hematopoiesis-related genes showed that IL-1β stimulation results in the down-regulation of Kitl (SCF) and Cxcl12b (Figure 6E). Cxcl12a was up-regulated early in the culture period and then down-regulated by 24 hours. No changes were detected in Il6 expression. In addition, IL-1β stimulation of UG26-1B6 cells did not change the expression level of the Il1r1 or the Il1rap but transiently induced Il1a and Csf1 (M-CSF) expression (not shown). No expression of Il1b was detected before or after IL-1β stimulation (not shown). These AGM stromal cell data, together with the IL-1β–induced changes observed in downstream effectors in AGM explants, suggest a complex subtle regulation of molecules and interacting cell types in the AGM to control hematopoietic cell growth and migration.
Discussion
We have shown here that the well-known adult cytokine, IL-1, and molecules essential in its signaling pathway are expressed and active in the hematopoietic system of the mouse AGM, suggesting that IL-1 signaling plays a role in early hematopoietic cell regulation. This regulation is complex, affecting myeloid lineage cells and progenitors, and HSCs. This was unexpected because gene-targeting experiments showed no obvious phenotype in adult mice. IL-1 (α and β) and IL-1RI–deficient mice are viable and under steady-state conditions do not display an obvious hematopoietic phenotype.41,42 However, on immune challenge, inflammatory responses and cytokine expression are reduced in IL-1RI–deficient mice. Our demonstration of IL-1 signaling in the AGM hematopoietic compartment supports the notion that tissue development in the embryo and repair resulting from trauma in the adult may be regulated similarly.
IL-1 affects growth and/or differentiation of AGM myeloid lineage cells and HSCs
In AGM explant cultures, we found that the addition of exogenous IL-1 increases the number of granulocyte and macrophage progenitors, whereas Il1r1−/− AGM explants contain fewer of these progenitors. Exogenous IL-1 addition to AGM explant cultures probably supplements a growth affect on AGM progenitors mediated by IL-1 produced endogenously (Figure 6). The decrease in the absolute numbers of CFU-G and CFU-M in Il1r1−/− AGM explants, compared with Il1r1+/+ AGM explant cultures, demonstrates that IL-1RI–mediated signaling is normally active in this tissue. Our data suggest that IL-1 is acting on the level of these hematopoietic progenitors; some progenitor expansion does occur in Il1r1−/− AGM explants compared with uncultured Il1r1−/− AGMs, albeit to a lesser extent than in Il1r1+/+ AGM explants. Thus, IL-1RI–mediated signaling is not the only signaling pathway affecting these cells.
Curiously, in directly isolated Il1r1−/− AGMs, CFU-M and CFU-G numbers and hematopoietic cell (c-kit+ and CD45+) numbers were greatly increased, compared with directly isolated Il1r1+/+ AGMs. Other hematopoietic progenitors (BFU-E, CFU-GM, CFU-GEMM) and HSCs (activity) were largely unaffected or slightly decreased in number, demonstrating that IL-1RI–mediated signaling in vivo acts predominantly to limit the growth of granulocyte and macrophage progenitors. The slight decrease in CFU-GEMM numbers and HSC activity may suggest a positive role for IL-1RI–mediated signaling in maintaining these cells (at least some of them) in an undifferentiated state. Clearly, normal AGM hematopoiesis requires a delicate balance between the level of IL-1 and IL-1RI–mediated signaling. A recent study illustrates the importance of a balanced IL-1 response in IKKβ-deficient mice. These mice have increased IL-1β secretion because of a lack of NF-κB–mediated negative feedback signaling and thus are highly susceptible to death from endotoxic shock.43
In the adult, IL-1 is known to affect several cellular processes, including proliferation, differentiation, and apoptosis3,44,45 (reviewed by Dinarello2 ). In the case of AGM hematopoietic progenitors in explant cultures, IL-1 probably functions as a proliferation factor. In addition, our findings that IL-1β induces increases in the number of Mac1+ cells and CFU-M in AGM explants support a role for IL-1β in differentiation. In other experiments (data not shown), we found that there was no consistent effect of IL-1 on the viability of c-kit+ cells, and IL-1 does not appear to affect changes in the survival/apoptosis of hematopoietic progenitors in the AGM region, although rare, immature AGM progenitors could be affected. We did observe a decrease in long-term repopulating HSCs and CFU-GEMM in AGM explants treated with a high dose of IL-1β (10 ng/mL). The loss of immature cell function could be the result of enhanced differentiation of these cells (only in some HSCs because Il1r1−/− AGM HSCs can be serially transplanted and thus self-renew) and is consistent with reports that IL-1 dosage differentially affects BM HSCs.46
Part of this complexity of regulation may be the result of the functional redundancy between IL-1 and IL-18 found in the adult.47,48 The fact that no Il18 and very low levels of Il18r1 are expressed in the wild-type AGMs and the IL-18–signaling pathway appears to be unaffected in Il1r1−/− E11 AGMs suggests that only the IL-1–signaling pathway is active within this tissue and that IL-1–mediated regulation of AGM hematopoiesis is both cell context- and dose-dependent.
Possible roles for IL-1 in hematopoietic cell migration and homing in the embryo
In the adult, IL-1 has also been implicated in the mobilization of HSCs from the adult BM to the peripheral blood.13 It acts on the vascular endothelium to up-regulate cell adhesion molecules for recruitment of leukocytic cells and induces the expression of chemo-attractant cytokines, such as IL-8 in epithelial and fibroblast cells.2,49 Moreover, it enhances the adhesion of CD34+ BM cells to BM vascular endothelial cells.14 In the embryo, whereas the AGM generates and maintains HSCs, the migration of HSCs to other hematopoietic tissue rudiments (FL) must occur for the development of the adult hematopoietic system. Similar to the findings that IL-1 induces MMP9 expression in human umbilical vein endothelial cells and vascular smooth muscle cells,17,50 we found that Mmp9 was up-regulated in cultures of AGM explants containing IL-1β (Figure 6B). Under these conditions, we also observed the down-regulation of Cxcl12 (SDF1-α and -β; Figure 6B), factors that were shown to be required for normal BM hematopoiesis but not for normal FL hematopoiesis.51 Interestingly, we found that Cxcl12b (and Mmp9) are up-regulated in vivo in Il1r1−/− AGMs (Figure 6D). Further studies are needed to reveal what AGM subpopulations are affected and whether these changes affect hematopoietic migration/mobilization in the embryo.
Does IL-1 act directly on AGM hematopoietic cells or cells of the microenvironment?
In immunostained AGM sections, IL-1R–expressing cells are localized mainly on the ventral side of the dorsal aorta and appear to be mainly endothelial and mesenchymal, with some low-level–expressing hematopoietic cells. We have enhanced the IL-1RI signal in immunostainings of AGM sections with a biotin-streptavidin step. Flow cytometric analyses confirm a small number of IL-1RI+CD45+ and IL-1RI+c-kit+ hematopoietic cells in the AGM, and these cells express IL-1RI to low levels compared with AGM endothelial or mesenchymal cells. In multicolor flow cytometric studies, it is highly probable that we missed some IL-1RI low-expressing AGM (hematopoietic) cells because a directly conjugated fluorescent anti-IL-1RI antibody was used. Nonetheless, IL-1 may act directly on some AGM hematopoietic cells to affect hematopoiesis.
Alternatively and/or additionally, IL-1 could act via the induced expression of other proliferation/differentiation factors by IL-1RI–positive endothelial and/or mesenchymal cells in the AGM. RT-PCR analysis of AGM tissues after explant culture did not reveal a clear change in the expression of hematopoietic growth factors. However, the UG26-1B6 stromal cell line showed IL-1–induced changes in the expression of the Kitl hematopoietic regulator, demonstrating that IL-1 does affect the hematopoietic microenvironment. The fact that all tested AGM stromal cell lines express IL-1RI and that IL-1RI expression levels influence HSC support capacity underlines this notion.40
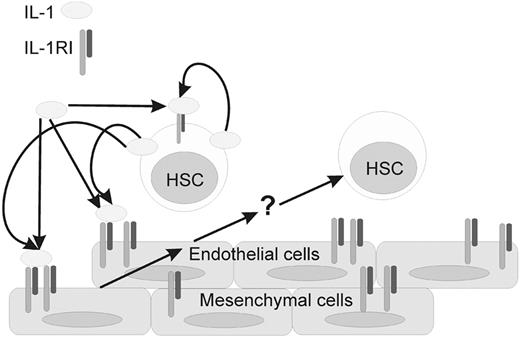
E11 AGM HSCs and endothelial cells, but not mesenchymal cells, produce IL-1 in vivo (high and low levels, respectively; Figure 3B). Hence, IL-1 signaling may occur through direct interactions of the IL-1–producing aortic endothelial or hematopoietic cells with the IL-1R+ stromal microenvironment or through secretion of IL-1 through the AGM interstitium. The IL-1–signaling cascade is then triggered in stroma (mesenchyme and/or endothelium) to effect changes in the expression and elaboration of molecules regulating the proliferation, differentiation, and mobilization of AGM hematopoietic cells (Figure 7). Because there is no expression of the majority of IL-1–signaling molecules at E10, IL-1 probably does not play a role in hematopoietic stem/progenitor cell emergence. Our data show, for the first time, that IL-1 can act as a regulator of the earliest adult repopulating HSCs and myeloid cells in the mouse embryo. Future studies should further elaborate how this “adult” cytokine acts to regulate AGM hematopoiesis through proliferation, differentiation, and/or migration.
Model of IL-1–related interactions in the E11 mouse AGM region. The IL-1RI is expressed on endothelial, mesenchymal, and at lower levels on some HSCs. Receptor expression appears to be stable. The expression of IL-1 is variable, with high levels being produced by HSCs and other hematopoietic cells. Expression of IL-1 is low or negligible in the endothelial and mesenchymal compartment. IL-1 (from HSCs or other hematopoietic cells) is thought to interact with IL-1RI–expressing endothelial and/or mesenchymal cells on the ventral side of the E11 dorsal aorta. IL-1RI signaling results in the induction of unknown factor(s) (eg, some mobilization factors) indicated with a question mark (?) to modulate HSC maintenance and/or differentiation.
Model of IL-1–related interactions in the E11 mouse AGM region. The IL-1RI is expressed on endothelial, mesenchymal, and at lower levels on some HSCs. Receptor expression appears to be stable. The expression of IL-1 is variable, with high levels being produced by HSCs and other hematopoietic cells. Expression of IL-1 is low or negligible in the endothelial and mesenchymal compartment. IL-1 (from HSCs or other hematopoietic cells) is thought to interact with IL-1RI–expressing endothelial and/or mesenchymal cells on the ventral side of the E11 dorsal aorta. IL-1RI signaling results in the induction of unknown factor(s) (eg, some mobilization factors) indicated with a question mark (?) to modulate HSC maintenance and/or differentiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all laboratory members for helpful discussions and technical support, especially K. van der Horn; Dr C. Durand for AGM cDNAs; Dr D. Meijer for antitubulin antibody; and R. van der Linden and Dr C. Robin for flow cytometry.
This work was supported by the National Institutes of Health (R37 DK51077), the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO; VICI 916.36.601), the Netherlands Besluit Subsidies Investeringen Kennisinfrastructuur (BSIK; Award 03038), the Koningin Wilhemina Fonds (KWF) Dutch Cancer Society (2001-2442), and the Human Frontiers Science Program (RG0345/1999).
National Institutes of Health
Authorship
Contribution: C.O. performed research, analyzed data, and wrote the paper; E.H. and M.P. performed research and analyzed data; and E.D. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elaine Dzierzak, Department of Cell Biology and Genetics, Erasmus University MC, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: e.dzierzak@erasmusmc.nl.
References
Author notes
*C.O., E.H., and M.P. contributed equally and are considered first authors.


![Figure 4. IL-1β increases hematopoietic cells in the E11 AGM region. E11 AGM tissues were cultured for 3 days in the presence or absence of different doses of IL-1β. (A) Flow cytometric analysis showing the absolute number of cells per AGM positive for CD45, c-kit, or Mac1 after AGM explant culture (n = 5). (B) Number of CFU-G, CFU-M, and CFU-GEMM per E11 AGM explant cultured for 3 days in the presence of 0, 1, or 10 ng/mL IL-1β. Colonies were scored in triplicate cultures after 7 days of methylcellulose culture (n = 2). (C) Percentage of adult recipient mice repopulated with donor E11 AGM cells. E11 AGM explants were cultured in 0, 1, or 10 ng/mL IL-1β for 3 days, and cells were injected into irradiated recipients (1 embryo equivalent [ee]). At 4 months after transplantation, recipient peripheral blood DNA was analyzed for donor hematopoietic chimerism by semiquantitative PCR. Only mice with more than 10% donor chimerism were considered repopulated. Each column represents the number of mice repopulated per number of recipients transplanted (13 of 24, 9 of 13, and 4 of 12) with AGM explant cells cultured in 0, 1, and 10 ng/mL IL-1β, respectively). Combined results of 8 separate transplantation experiments. The error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/13/10.1182_blood-2007-12-123836/4/m_zh80240827640004.jpeg?Expires=1764967712&Signature=z4JZh8BCM5EuiJzsCBU3bMqLavZ1WWWcnLUQ792TQo4SYstWGhfBDISnkCWxiMcVtIL5~~RdgWmel7al7BhImCLR2GY46CSnSTM8Sg7934ty9KUtbr5dzCCajhPHej2K4VR10jdZwiOD8DRh4KNIVHVZSZx5SrglMvL-kONojU7nbWNAhT6HxATNrV1x~jrfgWk3xJMUuHZIMHTqANNDv0IrtAbN1JpmEdYLIxyX29hsr8QBKdYU1VG6cAlKB6k1VfrsQtpAMNSlma4BKg2cMSE2~smhhPdr7f~VVlYfThfre4wiL-QSTj2ZO7YIZFj0EZSJnXsQSVmb8F-cGXVoMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. IL-1R signaling affects AGM HSCs. (A) Flow cytometric analysis of freshly isolated E11 Il1r1+/+ and Il1r1−/− AGM cells showing the absolute number of c-kit+, CD45+, CD34+c-kit+, or CD34−Mac1+ cells per AGM (n = 3). (B) Flow cytometric analysis of E11 Il1r1+/+ and Il1r1−/− AGM explants showing absolute numbers of c-kit+, CD45+, or Mac1+ cells per AGM explant (n = 4-5). (C) Number of CFU total, CFU-M, BFU-E, CFU-G, CFU-GM, and CFU-GEMM per freshly isolated E11 Il1r1+/+ and Il1r1−/− AGM. Colonies from triplicate cultures were scored after 7 days of methylcellulose culture (n = 3, cells from 14 Il1r1+/+ and 15 Il1r1−/− AGMs). (D) Number of CFU total, CFU-M, BFU-E, CFU-G, CFU-GM, and CFU-GEMM per E11 Il1r1+/+ and Il1r1−/− AGM after 3 days of explant culture. Colonies from triplicate cultures were scored after 7 days of methylcellulose culture (n = 3, cells from 8 Il1r1+/+ and 8 Il1r1−/− AGMs). (E) Percentage of adult recipient mice repopulated with HSCs from Il1r1+/+ and Il1r1−/− AGM regions either directly transplanted (direct) or transplanted after 3 days of explant culture (explant). E11 AGM cells (1 and 0.3 or 1 and 0.2 embryo AGM tissue equivalents [ee]) were transplanted into irradiated adult recipients. At 4 months after transplantation, peripheral blood DNA of recipients was analyzed for donor hematopoietic chimerism by semiquantitative PCR. Only mice with more than 10% donor chimerism were considered repopulated. Each column represents the number of mice repopulated per number of recipients transplanted (the numbers for the Il1r1+/+ and Il1r1−/− columns are, respectively, 5 of 7 and 3 of 8 for 1 ee direct transplantation, 1 of 6 and 0 of 8 for 0.3 ee direct transplantation, 2 of 2 and 5 of 5 for 1 ee explant transplantation, and 8 of 10 and 2 of 10 for 0.2 ee explant transplantation). Combined results of 4 separate transplantation experiments. The error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/13/10.1182_blood-2007-12-123836/4/m_zh80240827640005.jpeg?Expires=1764967712&Signature=lD1IBGPz6G27EJ03OjmH~HRcQa6RtNWga2opF4WvqLMEb6X4R8SQPamWAKQookNkXh-GAm5UJuRTQ5EmB2sxnAySyn8MBxHEdCZvkNFBss256Nm9NaVhtJn8giE8nFHu~Nq9B2cgnBejVLHLzbTnALt~TeyqPHKTS3mkOylXTuVV41AK8xsLDxMvrzryUK1EGfxGwGRZio9HxceZ0Ozz1RNURXRsDFg3HJFnLZ-FGQqnrI4bkEBqSWAJ2MrFNdgfJFmnn-ikzcB0wCyeGsEjfOw4r1eYZ7iWl9dDH9g-eH7yLnlFSZOz3LBuS0px-Y3ivAVefgIbNTDggmHNxxLYtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal