Abstract
Neutrophils recruited from the blood are key players in the innate immune response. Selectins play critical roles in neutrophil recruitment by mediating their tethering and rolling in inflamed venules. While the roles of P- and E-selectin in this process are well established, the mechanisms of L-selectin–mediated neutrophil recruitment remain elusive. One proposal is that tethering is mediated by L-selectin on flowing neutrophils interacting with P-selectin glycoprotein ligand-1 (PSGL-1) on adherent neutrophils. To clarify whether L-selectin–mediated neutrophil recruitment depends entirely on PSGL-1, we examined the impact of L-selectin deficiency in mice with a PSGL-1–deficient background. L-selectin and PSGL-1 double-knockout mice exhibited a higher increase in their peripheral blood neutrophil count and a worse defect in neutrophil recruitment into the inflamed peritoneum than PSGL-1–deficient mice. Intravital microscopy of inflamed cremaster muscle venules showed that L-selectindeficiency or antibody blockade of L-selectin reduced the residual leukocyte rolling in PSGL-1–deficient mice. Flow cytometric analyses showed that the endothelial cells from the cremaster muscle bound L-selectin in a PSGL-1–independent manner. These results provide evidence for the existence of an L-selectin ligand distinct from PSGL-1 in inflammation and indicate that such a ligand is expressed on endothelial cells, promoting neutrophil rolling in vivo.
Introduction
Neutrophils are recruited from the blood into sites of infection or injury, where they play critical roles in host defense.1 Neutrophil recruitment from the blood into tissues is initiated by the tethering of neutrophils to the vessel wall and their rolling along the endothelial surface. These initial steps are mediated by the selectin family of adhesion molecules.2 P-selectin (CD62P) and E-selectin (CD62E) expressed on activated endothelium are the primary mediators of neutrophil rolling in inflamed venules.3 Mice deficient in P-selectin show a striking absence of neutrophil rolling and a reduction in neutrophil migration early in the inflammatory response, which is restored at later times.4 Mice deficient in both P- and E-selectin show severely impaired neutrophil rolling and migration, even at later times.5,6
While L-selectin (CD62L), which is expressed by most leukocytes, is well known as a lymphocyte homing receptor that mediates the recruitment of lymphocytes to secondary lymphoid organs,7 it is also involved in neutrophil trafficking. This was initially indicated by the inhibitory effect of the anti–L-selectin mAb MEL-14 on neutrophil migration from the blood into sites of acute inflammation in the skin.8 The involvement of L-selectin in neutrophil migration was further indicated by the finding that significantly fewer neutrophils are recruited into the inflamed peritoneum of L-selectin–deficient (L-selectin−/−) mice.9,10 Leukocyte rolling in L-selectin−/− mice, observed by intravital microscopy, is not altered in freshly exteriorized venules, but is significantly decreased by 1 hour or later,9,11 or after stimulation with tumor necrosis factor-α (TNF-α).12,13 In vitro, cytokine-activated human endothelial cells can bind human neutrophils, monocytes, and lymphocytes, and the binding is blocked by an anti–L-selectin mAb.14-16 In addition, transfection of a human endothelial cell line with α-1,3-fucosyltransferase (FucT) VII induces the expression of functional L-selectin ligands and sialyl LewisX (sLeX).17 These findings suggest the existence of inducible endothelial ligands for L-selectin. However, the molecular identity of endothelial L-selectin ligands at sites of inflammation remains unknown. sLeX-independent ligands such as heparan sulfate have also been described,18,19 although their physiological functions in L-selectin–mediated neutrophil trafficking in vivo remain to be clarified.
P-selectin glycoprotein ligand-1 (PSGL-1; CD162), a sialomucin expressed by most leukocytes, was originally identified as the major ligand for P-selectin.20 PSGL-1–deficient (PSGL-1−/−) mice show a defect in neutrophil rolling and migration into inflamed sites resembling that in mice deficient in P-selectin, supporting the view that the defect in PSGL-1−/− mice is largely attributable to the lack of PSGL-1 interaction with P-selectin.21 However, PSGL-1 is also capable of binding to E-selectin22,23 and L-selectin.24,25
In vitro studies show that neutrophils roll on other neutrophils bound to cytokine-activated endothelial cells and that this rolling is mediated by L-selectin on the rolling cells.26 Neutrophil rolling on adherent neutrophils is blocked by an anti–PSGL-1 mAb, indicating that PSGL-1 on the adherent neutrophils serves as a functional ligand for L-selectin.27 The interaction of flowing neutrophils with adherent neutrophils facilitates their subsequent direct interaction with activated endothelial cells or immobilized substrates in vitro.27,28 This “secondary tethering” appears to be important in larger venules and arterial vessels in vivo.29 Sperandio et al30 found that the L-selectin–dependent leukocyte rolling in inflamed venules is completely absent in PSGL-1−/− mice, concluding that L-selectin–dependent leukocyte rolling is mediated by PSGL-1 expressed on leukocytes or leukocyte fragments already adherent to the endothelium.
To gain insight into the mechanisms of L-selectin–mediated neutrophil recruitment, we investigated whether L-selectin's role was entirely dependent on PSGL-1, by examining the impact of L-selectin deficiency on neutrophil trafficking in mice with a PSGL-1−/− background. If L-selectin functions only by interacting with PSGL-1 during neutrophil trafficking, L-selectin deficiency in the PSGL-1−/− background would not cause a further defect over that seen when only PSGL-1 is deficient. We observed that in L-selectin and PSGL-1 double-knockout (DKO) mice, the neutrophil count in peripheral blood was elevated more than in mice deficient in PSGL-1 alone. In a thioglycolate-induced peritonitis model, neutrophil migration was more severely impaired in DKO mice than in PSGL-1−/− mice. Intravital microscopy showed that L-selectin deficiency or antibody blockade of L-selectin reduced neutrophil rolling in inflamed venules in the PSGL-1−/− mice. In addition, we showed that L-selectin bound directly to cremaster muscle endothelial cells in a PSGL-1–independent manner. These results point to the existence of an L-selectin ligand distinct from PSGL-1 in inflammation and indicate that such a ligand is directly expressed on endothelial cells, promoting neutrophil rolling in vivo.
Methods
Mice
C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). PSGL-1−/− mice were produced as described previously21 and provided by Dr B. Furie (Harvard Medical School, Boston, MA). L-selectin−/− mice were produced as described9 and provided by Dr S. Sato (Kanazawa University, Kanazawa, Japan). Mice deficient in FucT-IV and FucT-VII on a C57BL/6 background were produced as described31 and provided by Dr R. Kannagi (Aichi Cancer Center, Aichi, Japan). Both PSGL-1−/− and L-selectin−/− mice were backcrossed more than 6 generations onto the C57BL/6 genetic background. DKO mice were generated by breeding L-selectin−/− mice with PSGL-1−/− mice. All mice used were 6 to 10 weeks old. The mice were housed at the Institute of Experimental Animal Sciences at Osaka University Medical School. All studies and procedures were approved by the Ethics Review Committee for Animal Experimentation of the Osaka University Graduate School of Medicine.
Flow cytometry
mAbs used for flow cytometric analyses included anti–CD3-FITC (145-2C11; BD Biosciences, San Jose, CA), anti–CD4-allophycocyanin (APC) (RM4-5; BioLegend, San Diego, CA), anti–CD8-APC (53-6.7; eBioscience, San Diego, CA), anti–B220-PE (RA3-6B2; BD Biosciences), anti–Gr-1-FITC and -APC (RB6-8C5; BioLegend), anti–F4/80-PE(CI:A3-1; Caltag, Burlingame, CA), anti–CD31-APC (MEC 13.3; BD Biosciences), anti–CD45-FITC and -PE-Cy5 (30-F11; BD Biosciences), anti–CD62L-FITC (MEL-14; BioLegend), anti–CD162-PE (2PH1; BD Biosciences), anti–CD54-biotin (3E2; BD Biosciences), anti–CD102-biotin (3C4; BD Biosciences), anti–CD106-biotin (429; BD Biosciences), anti-CD144 (11D4.1; BD Biosciences), and anti–Δ-heparan sulfate (F69-3G10; Seikagaku, Tokyo, Japan). Cells were stained with mAbs for 30 minutes on ice, washed, and analyzed on a FACSCalibur (BD Biosciences). For biotinylated and nonlabeled mAbs, streptavidin-PE (BD Biosciences), FITC-labeled goat anti–rat IgG (Cappel, Aurora, OH), and FITC-labeled donkey F(ab′)2 anti–mouse IgG (Jackson ImmunoResearch, West Grove, PA) were used as secondary reagents. For whole-blood flow cytometry, blood was incubated with anti-CD16/CD32 (2.4G2; BD Biosciences) for 10 minutes at room temperature, and then incubated with mAbs for 30 minutes at room temperature. Red blood cells were lysed with BD FACS Lysing Solution. Remaining leukocytes were washed and analyzed.
To assess L-selectin binding, L-selectin–IgG chimeric protein (R&D Systems, Minneapolis, MN) was incubated with PE-labeled goat F(ab′)2 anti–human IgG (Fcγ-specific) (Jackson ImmunoResearch) at a 1:2 ratio for 1 hour on ice to generate a complex. Cells were incubated with the complex, washed, and then stained with the indicated mAbs. In some experiments, the complex was incubated with the anti–L-selectin mAb MEL-14 (BD Biosciences) or isotype control rat IgG2a (eBioscience) for 30 minutes on ice before added to the cells.
Peripheral blood counts
Blood was collected from the tail vein of 9-week-old male mice of each genotype. The blood was diluted in Türk stain solution (Nacalai Tesque, Kyoto, Japan), and the total leukocyte counts were determined using a hemocytometer. Flow cytometry of whole blood was performed to determine the fraction of each subset.
Cytokine assays
Serum granulocyte colony-stimulating factor (G-CSF), GM-CSF, and IL-3 concentrations were determined by Bio-Plex cytokine assay (Bio-Rad Laboratories, Hercules, CA). Serum IL-17 levels were determined by ELISA using a Quantikine Kit (R&D Systems).
Thioglycolate-induced peritonitis model
Mice were given intraperitoneally an injection of 1 mL 4% thioglycolate medium (Difco, Detroit, MI). At 4 and 8 hours after this stimulation, the mice were killed, and 8 mL phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), 0.5 mM EDTA, and 10 U/mL heparin was injected into the peritoneal cavity. After the peritoneal wall was gently massaged, the injected wash was withdrawn. Total cell numbers in the peritoneal lavage were determined with a hemocytometer. The peritoneal cells were stained with anti–Gr-1-APC and anti–F4/80-PE, and the percentage of neutrophils (Gr-1highF4/80low) and macrophages (Gr-1highF4/80high) was determined by flow cytometry. From the total cell count in the peritoneal lavage and the percentage of neutrophils and macrophages, the absolute number was calculated.
Competitive migration assays
Competitive neutrophil migration assays were performed according to the published protocol32 with slight modifications. Peripheral blood leukocytes were isolated from PSGL-1−/− and DKO mice. An aliquot was stained with anti–Gr-1-APC for neutrophil counting. The PSGL-1−/− and DKO leukocytes were labeled with CFSE or tetramethylrhodamine isothiocyanate (TRITC), respectively, for 5 minutes at room temperature, and mixed so that the ratio of DKO to PSGL-1−/− neutrophils would be approximately 1:1. The mixture of PSGL-1−/− and DKO leukocytes was injected via the tail vein into recipient DKO mice that had just received thioglycolate intraperitoneally (106 neutrophils of each genotype per mouse). At 4 and 8 hours after the injection, the peritoneal exudates and peripheral blood were harvested, stained with anti–Gr-1-APC, and analyzed by flow cytometry. Neutrophils were determined as side scatter (SSC)highGr-1high cells. The results are presented as the ratio of DKO to PSGL-1−/− neutrophils in the peritoneum and blood, divided by the DKO to PSGL-1−/− input ratio.
Leukocyte rolling in the mouse cremaster muscle
Mice were treated with an intrascrotal injection of mouse TNF-α (1 μg in 300 μL saline; R&D Systems) 2.5 hours and 6 hours before exteriorization of the cremaster muscle. Mice were anesthetized with an intraperitoneal injection of 600 mg/kg urethane (Sigma-Aldrich, St Louis, MO) and 60 mg/kg α-chloralose (Sigma-Aldrich). To maintain neutral fluid balance, 1 mL saline was administered intraperitoneally. The cremaster muscle was prepared for intravital microscopy as described by Ley et al.11 The cremaster preparation was superfused with thermocontrolled (37°C) and aerated (5% CO2, 95% N2) bicarbonate-buffered saline throughout the experiment. Microvessel data were obtained using an intravital microscope (BX50; Olympus, Tokyo, Japan) fitted with a LUMPlan Fl (40×/0.80 numeric aperture [NA]) water-immersion objective. Leukocyte rolling in the venules was recorded for 65 to 70 seconds using an Ikegami CCD camera (model ICD-878; Tokyo, Japan) connected through a computer to a Panasonic video recorder (model DMR-E250V; Kadoma, Japan). The centerline red blood cell velocity in each venule was measured in real time with a dual photodiode velocimeter running a digital cross-correlation program (Microvessel Velocity OD-RT; CircuSoft Instrumentation, Hockessin, DE). Blood samples were taken to determine the systemic leukocyte counts. After the start of the cremaster surgery, data were acquired for 40 minutes. In experiments using neutralizing mAbs, the mice received 30 μg of the anti–E-selectin mAb 9A9 (kindly provided by B. Wolitzky, Coelacanth, East Windsor, NJ), 50 μg of the F(ab′)2 fragments generated from the anti–L-selectin mAb MEL-14 by immobilized pepsin (Pierce, Rockford, IL), or isotype controls, just before exteriorization of the cremaster muscle. Video recordings from the intravital microscopy experiments were analyzed as described previously.21
Isolation of cremaster muscle endothelial cells
Mice were either unstimulated or stimulated with an intrascrotal injection of TNF-α (1 μg in 300 μL saline). Then, 2.5 hours later, the cremaster muscle was removed, cut into small pieces, and incubated in RPMI 1640 containing 10% FCS, 400 U/mL collagenase D (Roche, Penzberg, Germany), and 10 μg/mL DNase I (Roche) with continuous stirring at 37°C for 1 hour. EDTA was added (10 mM final concentration), and the cell suspension was incubated for 5 additional min at 37°C. The resulting cell populations were filtered through a 100-μm strainer (BD Falcon, San Jose, CA) and then enriched for endothelial cells by centrifugation on 20%/50% Percoll (GE Healthcare, Uppsala, Sweden). The cells at the interface were collected and used for flow cytometry. In some experiments, the cells were pretreated at 37°C for 1 hour with 50 mU/mL heparitinase I and II (Seikagaku) before staining.
Statistical analysis
Data are presented as the mean plus or minus SEM. Statistical analyses were performed using the 2-tailed Student t test.
Results
Generation of mice lacking both L-selectin and PSGL-1
To investigate whether L-selectin–mediated neutrophil trafficking depends entirely on PSGL-1, mice deficient in both L-selectin and PSGL-1 were generated by crossbreeding L-selectin−/− mice with PSGL-1−/− mice. To confirm that the DKO mice were deficient in the cell-surface expression of L-selectin and PSGL-1, leukocytes from the blood of WT, L-selectin−/−, PSGL-1−/−, and DKO animals were stained with the anti–L-selectin mAb MEL-14 and the anti–PSGL-1 mAb 2PH1, and analyzed by flow cytometry. Leukocytes from DKO mice showed no specific staining for either L-selectin or PSGL-1 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), confirming the null mutations at both loci. The expression level of PSGL-1 in L-selectin−/− mice and L-selectin in PSGL-1−/− mice was comparable to that in WT mice (Figure S1). The expression of other adhesion molecules, including integrin chains αL (CD11a), αM (CD11b), β2 (CD18), β1 (CD29), α4 (CD49d), and β7, as well as CD44, on neutrophils and lymphocytes was also comparable among the 4 genotypes (data not shown). DKO mice developed normally, and their postnatal growth rate was comparable to that of WT and single-deficient mice. Both sexes were fertile. There were no obvious indications of pathology or disease susceptibility for any of the mice up to 1 year of age.
DKO mice exhibit more prominent neutrophilia than PSGL-1−/− mice
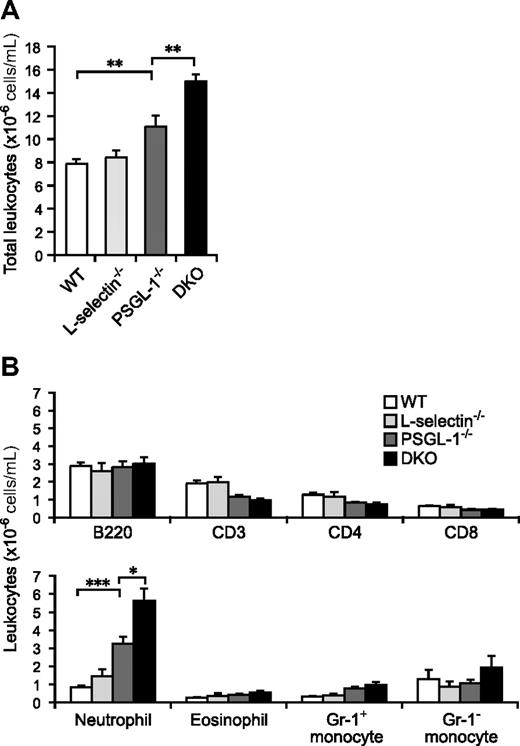
Although the total leukocyte count in peripheral blood was not significantly different between WT and L-selectin−/− mice, the PSGL-1−/− mice showed a slightly increased count (1.4-fold relative to WT mice; Figure 1A). The loss of both L-selectin and PSGL-1 led to a further elevation in the circulating leukocyte count (1.9-fold relative to WT mice), which was a significantly higher count than in the PSGL-1−/− mice (Figure 1A). To clarify which subsets of leukocytes were increased in the DKO mice, the number of cells in each subset was determined by flow cytometry. Neutrophils were 3.9-fold more frequent in PSGL-1−/− mice than in WT mice, and a 6.7-fold increase, compared with WT mice, was seen in DKO mice (Figure 1B). Eosinophil and Gr-1+ monocyte counts also tended to be higher in DKO mice than in WT mice (Figure 1B). While the B-lymphocyte counts were not significantly different among the 4 genotypes, the T-lymphocyte counts were slightly decreased in the PSGL-1−/− and DKO mice (Figure 1B). Thus, the elevation of the total leukocyte counts in DKO mice was mostly attributable to the increased neutrophil counts. The higher neutrophil count in DKO mice compared with PSGL-1−/− mice suggests that L-selectin is involved in neutrophil homeostasis independent of PSGL-1.
Peripheral blood leukocyte counts. (A) Total leukocyte counts. Blood was collected from the tail vein of 6 male mice of each genotype. The total leukocytes were counted on a hemocytometer. (B) Leukocyte subset counts. Flow cytometry was used to determine the fraction of each subset. Neutrophils were determined as Gr-1highF4/80low cells, eosinophils as Gr-1intermediateF4/80high cells (these cells are also very high in SSC), and monocytes as Gr-1highF4/80high and Gr-1-F4/80high cells. Values are means plus or minus SEM; *P < .05; **P < .01; ***P < .001.
Peripheral blood leukocyte counts. (A) Total leukocyte counts. Blood was collected from the tail vein of 6 male mice of each genotype. The total leukocytes were counted on a hemocytometer. (B) Leukocyte subset counts. Flow cytometry was used to determine the fraction of each subset. Neutrophils were determined as Gr-1highF4/80low cells, eosinophils as Gr-1intermediateF4/80high cells (these cells are also very high in SSC), and monocytes as Gr-1highF4/80high and Gr-1-F4/80high cells. Values are means plus or minus SEM; *P < .05; **P < .01; ***P < .001.
Serum G-CSF, IL-17, and GM-CSF levels are most prominently elevated in DKO mice
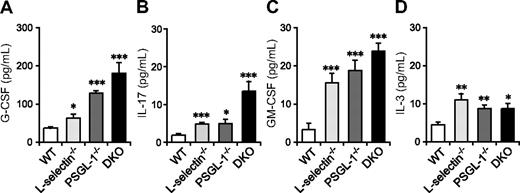
Neutrophilia is observed in many mutant mice deficient in leukocyte adhesion molecules and is associated with increased granulopoiesis through G-CSF.33 G-CSF is produced in response to various stimuli, including IL-17, GM-CSF, and IL-3. GM-CSF and IL-3 are also involved in hematopoiesis at the early stages of lineage commitment.34 To investigate whether the elevation in these cytokines correlates with the neutrophilia of DKO mice, we measured their levels in serum. Serum G-CSF levels were slightly elevated in L-selectin−/− mice compared with WT mice, moderately elevated in PSGL-1−/− mice, and most prominently elevated in DKO mice (Figure 2A). IL-17 and GM-CSF levels were also increased in these mutant mice, with DKO mice showing the highest levels (Figure 2B,C). Serum IL-3 levels were also increased in L-selectin−/−, PSGL-1−/−, and DKO mice, but there were no significant differences among these 3 genotypes (Figure 2D). Thus, the elevated neutrophil count in DKO mice appears to correlate with increased levels of G-CSF, IL-17, and GM-CSF. The fact that the most prominent increase in these cytokines was in DKO mice also supports the hypothesis that L-selectin functions independent of PSGL-1 in neutrophil homeostasis.
Serum levels of G-CSF, IL-17, and GM-CSF are elevated in mice deficient in both L-selectin and PSGL-1. Serum levels of G-CSF (A), GM-CSF (C), and IL-3 (D) were determined by a Bio-Plex cytokine assay. Serum levels of IL-17 (B) were determined by ELISA. Data are expressed as means plus or minus SEM from 8 mice per group. *P < 0.05; **P < 0.01; ***P < .001 versus WT.
Serum levels of G-CSF, IL-17, and GM-CSF are elevated in mice deficient in both L-selectin and PSGL-1. Serum levels of G-CSF (A), GM-CSF (C), and IL-3 (D) were determined by a Bio-Plex cytokine assay. Serum levels of IL-17 (B) were determined by ELISA. Data are expressed as means plus or minus SEM from 8 mice per group. *P < 0.05; **P < 0.01; ***P < .001 versus WT.
Neutrophil migration during experimental peritonitis is more severely impaired in DKO mice than in PSGL-1−/− mice
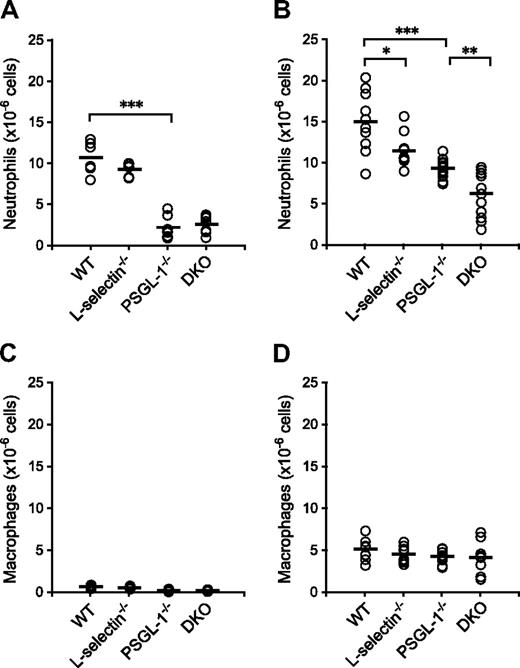
To examine neutrophil migration into sites of inflammation, chemical peritonitis was induced in WT, L-selectin−/−, PSGL-1−/−, and DKO mice by thioglycolate given as an intraperitoneal injection. The number of neutrophils migrating into the peritoneal cavity was reduced in the PSGL-1−/− and DKO mice (79% and 75% reduction relative to WT mice, respectively) 4 hours after the thioglycolate injection, but the difference between WT and L-selectin−/− mice and between PSGL-1−/− and DKO mice was not apparent at this time point (Figure 3A). In accordance with the published results,9,10 the number of migrated neutrophils was reduced in the L-selectin−/− mice (24% reduction relative to WT mice) 8 hours after the thioglycolate injection (Figure 3B). In PSGL-1−/− mice, the number of migrated neutrophils was reduced by 38% compared with WT mice (Figure 3B), which also confirms previous results.21 Importantly, the DKO mice showed a 58% reduction in the number of neutrophils migrating into the peritoneal cavity compared with WT mice, which was a more profound decrease than that seen in the PSGL-1−/− mice (Figure 3B). At these time points, more than 60% of the cells isolated from the peritoneal cavity were neutrophils in WT mice, and no differences were not yet observed among the 4 genotypes in the number of macrophages (Figure 3C,D), which are typically recruited 24 to 48 hours after thioglycolate injection.10 The decreased number of neutrophils migrating into the peritoneal cavity in these mutant mice is not due to accelerated apoptosis of neutrophils, since peripheral blood neutrophils from L-selectin−/− mice underwent apoptosis similarly to WT neutrophils, and those from PSGL-1−/− and DKO mice were even delayed in apoptosis (Figure S2). These results together indicate that both L-selectin and PSGL-1 are involved in neutrophil migration. The observation that L-selectin deficiency further impaired neutrophil migration in the PSGL-1−/− background suggests that the role of L-selectin in neutrophil migration is not entirely PSGL-1 dependent.
Neutrophil migration in thioglycolate-induced peritonitis. WT, L-selectin−/−, PSGL-1−/−, and DKO mice given an intraperitoneal injection of thioglycolate. Absolute neutrophil counts (A,B) and macrophage counts (C,D) in the peritoneal exudates were determined 4 hours (A,C) and 8 hours (B,D) after the thioglycolate injection. Each circle represents the value of an individual mouse. Bars express the mean. *P < .05; **P < .01; ***P < .001.
Neutrophil migration in thioglycolate-induced peritonitis. WT, L-selectin−/−, PSGL-1−/−, and DKO mice given an intraperitoneal injection of thioglycolate. Absolute neutrophil counts (A,B) and macrophage counts (C,D) in the peritoneal exudates were determined 4 hours (A,C) and 8 hours (B,D) after the thioglycolate injection. Each circle represents the value of an individual mouse. Bars express the mean. *P < .05; **P < .01; ***P < .001.
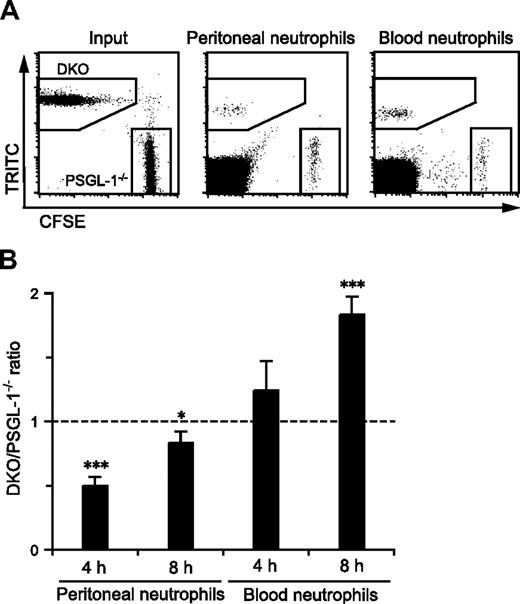
PSGL-1−/− and DKO mice exhibited different states of granulopoiesis with different levels of neutrophil pools in the circulating blood, which may have affected neutrophil migration from the blood into inflamed sites. Thus, to compare more directly the migration efficiency of PSGL-1−/− and DKO neutrophils in the same environment, competitive migration assays were performed. A previous study showed that fluorescently labeled peripheral blood neutrophils could migrate into the inflamed peritoneum of recipient mice.32 Peripheral blood leukocytes from PSGL-1−/− and DKO mice were individually labeled with either CFSE or TRITC, and the mixture of cells was injected intravenously into DKO mice that had just received an intraperitoneal injection of thioglycolate. DKO mice were used as recipients to exclude any possible effects of L-selectin and PSGL-1 expressed on endogenous leukocytes on the migration of the injected neutrophils. As shown in Figure 4A, the peritoneal exudate and peripheral blood from the recipient mice contained fluorescently labeled neutrophils of both the PSGL-1−/− and DKO genotypes. The ratio of DKO to PSGL-1−/− neutrophils that migrated into the inflamed peritoneum was 0.5 at 4 hours after injection (Figure 4B), indicating that the DKO neutrophils were more defective than PSGL-1−/− neutrophils in migrating into the inflamed site. At 8 hours after injection, the difference in migration into the peritoneal cavity between DKO and PSGL-1−/− neutrophils was reduced (Figure 4B). On the other hand, more DKO neutrophils accumulated in the blood than PSGL-1−/− neutrophils, especially at 8 hours after injection (Figure 4B). Switching the tracking fluorescent dyes between PSGL-1−/− and DKO cells yielded similar results (data not shown). These results demonstrate that L-selectin functions in neutrophil migration in a PSGL-1–independent manner.
Competitive migration of PSGL-1−/− and DKO neutrophils into the inflamed peritoneum. Peripheral blood leukocytes were isolated from PSGL-1−/− and DKO mice and labeled with CFSE and TRITC, respectively. The mixture of these cells was injected via the tail vein into recipient DKO mice that had just received thioglycolate intraperitoneally. After 4 and 8 hours, the blood and peritoneal exudate cells were collected, stained with anti–Gr-1-APC, and analyzed by flow cytometry. (A) Representative plots showing CFSE-labeled PSGL-1−/− neutrophils and TRITC-labeled DKO neutrophils in the input, and the peritoneal exudates and peripheral blood collected 4 hours after injection. (B) The ratio of DKO neutrophils to PSGL-1−/− neutrophils in the peritoneal exudates and peripheral blood normalized by the input ratio. Data are expressed as means plus or minus SEM from 3 mice. Results represent one of 3 similar experiments. *P < .05; ***P < .001.
Competitive migration of PSGL-1−/− and DKO neutrophils into the inflamed peritoneum. Peripheral blood leukocytes were isolated from PSGL-1−/− and DKO mice and labeled with CFSE and TRITC, respectively. The mixture of these cells was injected via the tail vein into recipient DKO mice that had just received thioglycolate intraperitoneally. After 4 and 8 hours, the blood and peritoneal exudate cells were collected, stained with anti–Gr-1-APC, and analyzed by flow cytometry. (A) Representative plots showing CFSE-labeled PSGL-1−/− neutrophils and TRITC-labeled DKO neutrophils in the input, and the peritoneal exudates and peripheral blood collected 4 hours after injection. (B) The ratio of DKO neutrophils to PSGL-1−/− neutrophils in the peritoneal exudates and peripheral blood normalized by the input ratio. Data are expressed as means plus or minus SEM from 3 mice. Results represent one of 3 similar experiments. *P < .05; ***P < .001.
Leukocyte rolling in inflamed venules is severely reduced in DKO mice
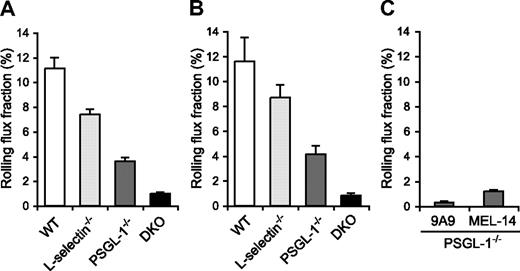
Since the migration of neutrophils is initiated by their tethering and rolling in inflamed postcapillary venules, we next examined leukocyte rolling in the TNF-α–treated postcapillary venules of the cremaster muscle by intravital microscopy in WT, L-selectin−/−, PSGL-1−/−, and DKO mice. The mice were treated with an intrascrotal injection of TNF-α 2.5 hours before cremaster muscle exteriorization. The microvessel and hemodynamic parameters were closely matched across the 4 genotype groups (Table 1). In agreement with the results of other investigators,12 the leukocyte rolling flux fraction in L-selectin−/− mice (7.4%) was lower than in WT mice (11.1%; Figure 5A, Videos S1,S2). In PSGL-1−/− mice, the leukocyte rolling flux fraction (3.6%) was also reduced (Figure 5A; Video S3), which is consistent with published results.21,35 Strikingly, DKO mice exhibited a pronounced defect in leukocyte rolling, with an average leukocyte rolling flux fraction of 1.0% (Figure 5A; Video S4). The mice were also treated with TNF-α 6 hours before cremaster muscle exteriorization. The microvessel and hemodynamic parameters were matched across the 4 genotype groups (Table 1). At this time point, too, the leukocyte rolling flux fraction in L-selectin−/− mice (8.7%) and PSGL-1−/− mice (4.1%) was lower than in WT mice (11.6%), and that in DKO mice (0.8%) was even more severely reduced (Figure 5B). These results demonstrate that both L-selectin and PSGL-1 contribute significantly to leukocyte rolling in inflamed venules. The more severe defect in leukocyte rolling in DKO mice compared with mice deficient in PSGL-1 alone indicates that L-selectin plays a critical role in leukocyte rolling in the absence of PSGL-1.
Hemodynamic and microvascular parameters of cremaster muscle venules in TNF-α–induced inflammation
| TNF-α treatment/mouse genotype . | Mice (n) . | Venules (n) . | Diameter, μm . | Centerline velocity, mm/s . | Wall shear rate, s−1 . |
|---|---|---|---|---|---|
| 2.5 hours | |||||
| WT | 5 | 35 | 36.2 ± 1.2 | 2.0 ± 0.1 | 590 ± 17 |
| L-selectin−/− | 6 | 49 | 36.5 ± 0.9 | 2.0 ± 0.1 | 589 ± 12 |
| PSGL-1−/− | 5 | 29 | 36.0 ± 1.5 | 1.9 ± 0.1 | 567 ± 15 |
| DKO | 4 | 41 | 35.6 ± 1.0 | 2.0 ± 0.1 | 590 ± 17 |
| 6 hours | |||||
| WT | 4 | 28 | 35.2 ± 1.1 | 1.7 ± 0.1 | 520 ± 25 |
| L-selectin−/− | 4 | 22 | 29.9 ± 1.6 | 1.4 ± 0.1 | 516 ± 28 |
| PSGL-1−/− | 3 | 24 | 29.2 ± 1.1 | 1.4 ± 0.1 | 522 ± 26 |
| DKO | 4 | 31 | 30.9 ± 1.4 | 1.5 ± 0.1 | 521 ± 29 |
| TNF-α treatment/mouse genotype . | Mice (n) . | Venules (n) . | Diameter, μm . | Centerline velocity, mm/s . | Wall shear rate, s−1 . |
|---|---|---|---|---|---|
| 2.5 hours | |||||
| WT | 5 | 35 | 36.2 ± 1.2 | 2.0 ± 0.1 | 590 ± 17 |
| L-selectin−/− | 6 | 49 | 36.5 ± 0.9 | 2.0 ± 0.1 | 589 ± 12 |
| PSGL-1−/− | 5 | 29 | 36.0 ± 1.5 | 1.9 ± 0.1 | 567 ± 15 |
| DKO | 4 | 41 | 35.6 ± 1.0 | 2.0 ± 0.1 | 590 ± 17 |
| 6 hours | |||||
| WT | 4 | 28 | 35.2 ± 1.1 | 1.7 ± 0.1 | 520 ± 25 |
| L-selectin−/− | 4 | 22 | 29.9 ± 1.6 | 1.4 ± 0.1 | 516 ± 28 |
| PSGL-1−/− | 3 | 24 | 29.2 ± 1.1 | 1.4 ± 0.1 | 522 ± 26 |
| DKO | 4 | 31 | 30.9 ± 1.4 | 1.5 ± 0.1 | 521 ± 29 |
Diameter, centerline velocity, and wall shear rate are presented as the mean plus or minus SEM of all the venules investigated.
Leukocyte rolling flux fraction in TNF-α–treated cremaster muscle venules. (A,B) Leukocyte rolling flux fraction in 2.5-hour (A) and 6-hour (B) TNF-α–treated cremaster muscle venules of WT, L-selectin−/−, PSGL-1−/−, and DKO mice. (C) Effect of anti–E-selectin and anti–L-selectin mAbs on leukocyte rolling in 2.5-hour TNF-α–treated cremaster muscle venules of PSGL-1−/− mice. Leukocyte rolling flux fractions were determined in PSGL-1−/− mice that had received the anti–E-selectin mAb 9A9, the F(ab′)2 fragments of the anti–L-selectin mAb MEL-14, or isotype controls.
Leukocyte rolling flux fraction in TNF-α–treated cremaster muscle venules. (A,B) Leukocyte rolling flux fraction in 2.5-hour (A) and 6-hour (B) TNF-α–treated cremaster muscle venules of WT, L-selectin−/−, PSGL-1−/−, and DKO mice. (C) Effect of anti–E-selectin and anti–L-selectin mAbs on leukocyte rolling in 2.5-hour TNF-α–treated cremaster muscle venules of PSGL-1−/− mice. Leukocyte rolling flux fractions were determined in PSGL-1−/− mice that had received the anti–E-selectin mAb 9A9, the F(ab′)2 fragments of the anti–L-selectin mAb MEL-14, or isotype controls.
The leukocyte rolling observed in the venules of TNF-α–treated PSGL-1−/− mice was largely E-selectin dependent, as injection of the anti–E-selectin mAb 9A9 decreased the leukocyte rolling flux fraction to 0.3% (Figure 5C). As described above, L-selectin deficiency in the PSGL-1−/− background resulted in a decrease in the leukocyte rolling flux fraction, to 1.0%. Similarly, blockade of L-selectin function by the F(ab′)2 fragments of the anti–L-selectin mAb MEL-14 in PSGL-1−/− mice led to a decrease in the leukocyte rolling flux fraction, to 1.2% (Figure 5C). In these experiments, intravenous injection of neither 9A9 nor MEL-14 F(ab′)2 fragments affected the circulating leukocyte counts, and isotype control mAbs rat IgG1 and the F(ab′)2 fragments of rat IgG2a had no effect on either the circulating leukocyte counts or leukocyte rolling in PSGL-1−/− mice (data not shown). These results suggest that L-selectin plays an important role in E-selectin–mediated leukocyte rolling independent of PSGL-1.
Endothelial cells from the cremaster muscle bind L-selectin
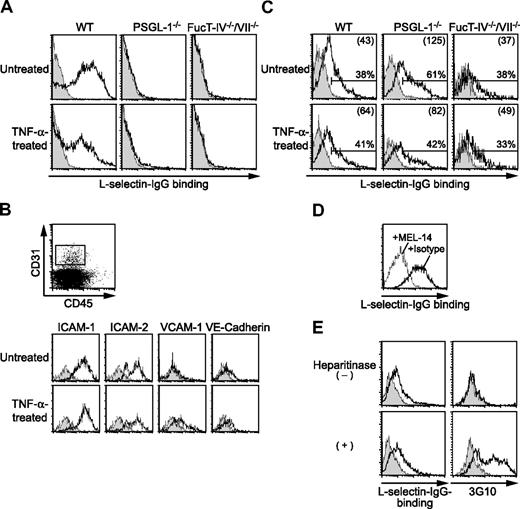
The finding of PSGL-1–independent L-selectin function suggested the existence of L-selectin ligands other than PSGL-1. We first sought to determine whether PSGL-1–independent L-selectin ligands are expressed on neutrophils. Flow cytometric analyses showed that L-selectin–IgG chimeric protein complexed with a secondary antibody bound to peripheral blood neutrophils from WT mice in a calcium-dependent manner (Figure 6A), while the control human IgG-secondary antibody complex did not bind at all (data not shown). In contrast, the binding of L-selectin–IgG to PSGL-1−/− neutrophils was mostly abolished (Figure 6A), indicating that PSGL-1 is the major L-selectin ligand on neutrophils. Peripheral blood neutrophils from mice treated 2.5 hours before with an intrascrotal injection of TNF-α also bound L-selectin in a PSGL-1–dependent manner (Figure 6A).
Cremaster muscle endothelial cells bind L-selectin. (A) L-selectin–IgG binding to neutrophils. Peripheral blood leukocytes from WT, PSGL-1−/−, and FucT-IV−/−/VII−/− mice treated with or without an intrascrotal injection of TNF-α were stained with L-selectin–IgG complexed with PE-labeled goat F(ab′)2 anti–human IgG with (shaded histograms) or without (open histograms) EDTA, and then stained with anti–Gr-1-APC. Cells were gated for neutrophils (SSChighGr-1high). (B) Expression of endothelial markers on cremaster muscle endothelial cells. Cremaster muscle was removed from WT mice treated with or without an intrascrotal injection of TNF-α. Single-cell suspensions enriched for endothelial cells by Percoll were stained with the indicated mAbs (open histograms) or isotype controls (shaded histograms) followed by secondary reagents together with anti–CD31-APC and anti–CD45-FITC or -PE-Cy5. Cells were gated on CD31+CD45−, as shown in the top panel. (C-E) L-selectin–IgG binding to cremaster muscle endothelial cells. Single-cell suspensions from the cremaster muscle were stained with L-selectin–IgG complexed with PE-labeled goat F(ab′)2 anti–human IgG and then stained with anti–CD31-APC and anti–CD45-FITC. Cells were gated on CD31+CD45−. (C) L-selectin–IgG binding to cremaster muscle endothelial cells from WT, PSGL-1−/−, and FucT-IV−/−/VII−/− mice treated with or without an intrascrotal injection of TNF-α. The cells were stained with (shaded histograms) or without (open histograms) EDTA. Percentages of L-selectin–binding cells are given. The numbers in parentheses indicate mean fluorescence intensity values. (D) Inhibition of L-selectin–IgG binding to cremaster muscle endothelial cells by the anti–L-selectin mAb MEL-14. The L-selectin–IgG secondary antibody complex was incubated with MEL-14 (dotted line) or isotype control (solid line) before added to the cells. (E) L-selectin–IgG binding to cremaster muscle endothelial cells treated with heparitinase. The cells were treated with heparitinase before being analyzed for L-selectin–IgG binding. The cells were stained with (shaded histograms) or without (open histograms) EDTA. The cells were also stained with (open histograms) or without (shaded histograms) the anti–Δ-heparan sulfate mAb 3G10 followed by FITC-labeled donkey anti–mouse IgG. Results represent one of 3 to 5 similar experiments.
Cremaster muscle endothelial cells bind L-selectin. (A) L-selectin–IgG binding to neutrophils. Peripheral blood leukocytes from WT, PSGL-1−/−, and FucT-IV−/−/VII−/− mice treated with or without an intrascrotal injection of TNF-α were stained with L-selectin–IgG complexed with PE-labeled goat F(ab′)2 anti–human IgG with (shaded histograms) or without (open histograms) EDTA, and then stained with anti–Gr-1-APC. Cells were gated for neutrophils (SSChighGr-1high). (B) Expression of endothelial markers on cremaster muscle endothelial cells. Cremaster muscle was removed from WT mice treated with or without an intrascrotal injection of TNF-α. Single-cell suspensions enriched for endothelial cells by Percoll were stained with the indicated mAbs (open histograms) or isotype controls (shaded histograms) followed by secondary reagents together with anti–CD31-APC and anti–CD45-FITC or -PE-Cy5. Cells were gated on CD31+CD45−, as shown in the top panel. (C-E) L-selectin–IgG binding to cremaster muscle endothelial cells. Single-cell suspensions from the cremaster muscle were stained with L-selectin–IgG complexed with PE-labeled goat F(ab′)2 anti–human IgG and then stained with anti–CD31-APC and anti–CD45-FITC. Cells were gated on CD31+CD45−. (C) L-selectin–IgG binding to cremaster muscle endothelial cells from WT, PSGL-1−/−, and FucT-IV−/−/VII−/− mice treated with or without an intrascrotal injection of TNF-α. The cells were stained with (shaded histograms) or without (open histograms) EDTA. Percentages of L-selectin–binding cells are given. The numbers in parentheses indicate mean fluorescence intensity values. (D) Inhibition of L-selectin–IgG binding to cremaster muscle endothelial cells by the anti–L-selectin mAb MEL-14. The L-selectin–IgG secondary antibody complex was incubated with MEL-14 (dotted line) or isotype control (solid line) before added to the cells. (E) L-selectin–IgG binding to cremaster muscle endothelial cells treated with heparitinase. The cells were treated with heparitinase before being analyzed for L-selectin–IgG binding. The cells were stained with (shaded histograms) or without (open histograms) EDTA. The cells were also stained with (open histograms) or without (shaded histograms) the anti–Δ-heparan sulfate mAb 3G10 followed by FITC-labeled donkey anti–mouse IgG. Results represent one of 3 to 5 similar experiments.
To investigate whether L-selectin ligands are directly expressed on endothelial cells, single cells were prepared from the cremaster muscle. Endothelial cells were identified as CD31+CD45− cells, which expressed other endothelial markers, such as intracellular adhesion molecule-1 (ICAM-1; CD54), ICAM-2 (CD102), and VE-cadherin (CD144) (Figure 6B). The expression level of vascular cell adhesion mlecule-1 (VCAM-1; CD106) was increased when the mice were treated 2.5 hours before with an intrascrotal injection of TNF-α (Figure 6B). The binding of L-selectin–IgG to these endothelial cells was examined. CD31+CD45− endothelial cells in the cremaster muscle from untreated WT mice as well as those treated 2.5 hours before with an intrascrotal injection of TNF-α bound L-selectin–IgG in a calcium-dependent manner (Figure 6C). The binding was almost completely abolished by the presence of the anti–L-selectin mAb MEL-14 (Figure 6D), confirming the specificity of the binding. Similar binding to endothelial cells from PSGL-1−/− mice was observed (Figure 6C), suggesting that L-selectin ligands other than PSGL-1 are expressed on the endothelial cells. No binding of control human IgG was observed for either WT or PSGL-1−/− endothelial cells (data not shown). These results suggest that L-selectin ligands distinct from PSGL-1 are expressed directly on endothelial cells.
L-selectin ligands expressed in the high endothelial venules (HEVs) of secondary lymphoid organs consist of a group of mucin-like glycoproteins that carry sulfated, sialylated, and fucosylated O-linked glycans.36 These HEV-borne L-selectin ligands require the concerted action of FucT-IV and FucT-VII.31 To investigate whether L-selectin ligands expressed on the cremaster muscle endothelial cells require FucT-IV and FucT-VII, we examined L-selectin binding to the cremaster muscle endothelial cells from mice deficient in both FucT-IV and FucT-VII (FucT-IV−/−/VII−/−). Although the binding of L-selectin–IgG to FucT-IV−/−/VII−/− neutrophils was completely abolished (Figure 6A), the endothelial cells from these mice bound L-selectin–IgG in a calcium-dependent manner (Figure 6C), suggesting that an L-selectin ligand expressed on cremaster muscle endothelial cells is molecularly different from HEV-borne L-selectin ligands.
Previous reports implicated heparan sulfate as an endothelial L-selectin ligand.18,19 To investigate whether heparan sulfate represents the L-selectin ligand on cremaster muscle endothelial cells, we treated cremaster muscle cells with heparitinase, which cleaves heparan sulfate, and examined L-selectin binding to these cells. Treatment of the cells with heparitinase induced the staining with the mAb 3G10, which recognizes heparan sulfate stubs exposed after cleavage of heparan sulfate (Figure 6E). This treatment, however, did not abolish the L-selectin–IgG binding to these cells (Figure 6E), suggesting that an L-selectin ligand on cremaster muscle endothelial cells is distinct from heparan sulfate.
Discussion
Several studies on the role of L-selectin in neutrophil trafficking have been reported, but the identity of the L-selectin ligands during inflammation has been unclear. A recent study using intravital microscopy demonstrated that L-selectin–dependent leukocyte rolling is mediated by PSGL-1 expressed on adherent leukocytes or leukocyte fragments.30 However, there are also reports that indicate L-selectin ligands are directly expressed on endothelial cells.14-17 In addition, there are reports indicating the existence of PSGL-1–independent L-selectin ligands on leukocytes.37,38 These findings prompted us to examine whether the role of L-selectin in neutrophil trafficking is entirely dependent on PSGL-1. We found that an L-selectin deficiency in the PSGL-1−/− background further increased the peripheral blood neutrophil counts and the serum levels of cytokines involved in neutrophil homeostasis, such as G-CSF. In addition, the L-selectin deficiency further impaired neutrophil migration into the inflamed peritoneum and dramatically decreased leukocyte rolling in TNF-α–treated venules in the PSGL-1−/− background. These results strongly suggest the existence of an L-selectin ligand distinct from PSGL-1, which plays an important role in neutrophil trafficking.
Although previous reports indicated the existence of PSGL-1–independent L-selectin ligands on human leukocytes,37,38 our data showed that PSGL-1−/− neutrophils did not bind L-selectin–IgG at all, indicating that PSGL-1 is the major L-selectin ligand on mouse neutrophils. Whether this is due to differences in animal species, cell types, or assays used remains to be clarified. In contrast, PSGL-1−/− endothelial cells bound L-selectin–IgG, suggesting that PSGL-1–independent L-selectin ligands are directly expressed on endothelial cells.
Sperandio et al30 reported that PSGL-1 accounts for all the L-selectin–dependent leukocyte rolling in the TNF-α–treated cremaster muscle model. The major difference between their study and ours is whether the L-selectin–dependent rolling requires E-selectin to be visualized. They investigated the L-selectin–dependent rolling in the presence of neutralizing antibodies against P-selectin and E-selectin, indicating that the rolling they studied is independent of P-selectin and E-selectin. Our intravital experiments showed that a blocking anti–L-selectin mAb reduced leukocyte rolling in the PSGL-1−/− mice, similar to the effect of L-selectin deficiency. On the other hand, leukocyte rolling in the TNF-α–treated venules of PSGL-1−/− mice was almost completely abrogated by an anti–E-selectin mAb, indicating that the rolling in PSGL-1−/− mice is largely mediated by E-selectin. These findings together suggest that L-selectin plays an important role in E-selectin–mediated leukocyte rolling independent of PSGL-1. The important role of L-selectin in this E-selectin–mediated process may raise the possibility that L-selectin functions as an E-selectin ligand, although this is unlikely, since L-selectin on mouse neutrophils does not bind E-selectin.39 Thus, L-selectin interaction with an endothelial ligand other than E-selectin probably initiates neutrophil tethering, which is then converted to E-selectin–mediated rolling.
L-selectin ligands expressed in the HEVs of secondary lymphoid organs consist of a group of mucin-like glycoproteins that carry sulfated, sialylated, and fucosylated O-linked glycans, collectively termed peripheral node addressin (PNAd).36 PNAd is also expressed on activated blood vessels at several sites of chronic inflammation.36 Although the molecular identity of an L-selectin ligand expressed on cremaster muscle endothelial cells is not known at present, this ligand may not depend on FucT-IV or FucT-VII, which are required for the L-selectin ligand activity of PNAd.31 In addition to PNAd, L-selectin can bind to sulfated glycosaminoglycans such as heparin, heparan sulfate, and chondroitin sulfate, in vitro.18,40,41 In a previous report, heparan sulfate deficiency in endothelial cells increased the rolling velocity of WT neutrophils, but not of L-selectin−/− neutrophils, in flow chamber assays in vitro, suggesting that endothelial heparan sulfate interacts with L-selectin.19 Our experiments showed that L-selectin binding to cremaster muscle endothelial cells was not abolished after treatment of the cells with heparitinase, which cleaves heparan sulfate, suggesting that an L-selectin ligand on cremaster muscle endothelial cells is distinct from heparan sulfate. In addition, this endothelial L-selectin ligand may be functionally different from HEV-expressed PNAd: HEV-expressed PNAd mediates both tethering and rolling, while the L-selectin ligand detected in this study appears inefficient in mediating rolling by itself. This proposal is based on the observation that leukocyte rolling in PSGL-1−/− mice was almost completely abrogated by an anti–E-selectin mAb, even though both L-selectin and an endothelial L-selectin ligand were expressed. Similarly, in noninflamed vessels, leukocytes do not roll, even though an L-selectin ligand is expressed on endothelial cells. We propose that L-selectin interaction with an endothelial L-selectin ligand is converted to leukocyte rolling only in inflamed venules, where E-selectin is expressed.
Our study demonstrated that leukocyte rolling was severely impaired in inflamed venules in the DKO mice. It should be noted that the DKO neutrophils expressed PSGL-1–independent E-selectin ligands such as E-selectin ligand-142 and CD44,43 yet these ligands do not appear to initiate E-selectin–mediated rolling efficiently in the absence of PSGL-1 and L-selectin. In addition, blocking both P- and L-selectin functions by gene targeting or antibody blockade severely impairs TNF-α–induced leukocyte rolling, even though E-selectin function is not blocked.11,12,44 These results suggest that E-selectin is inefficient in mediating rolling in the absence of P- and L-selectin. In these settings, both PSGL-1 and E-selectin are supposedly functional, but the severe impairment in leukocyte rolling in these mice suggests that, although PSGL-1 functions as one of the E-selectin ligands, the PSGL-1–E-selectin interaction by itself does not efficiently mediate tethering to initiate E-selectin–mediated rolling. Thus, it is likely that either PSGL-1's interaction with P-selectin or L-selectin's interaction with its ligand mediates leukocyte tethering as a prerequisite for E-selectin–mediated leukocyte rolling.
In conclusion, our study provides evidence for the existence of an L-selectin ligand distinct from PSGL-1, which is expressed on endothelial cells and plays an important role in neutrophil homeostasis and trafficking. Further investigation will be required to clarify the molecular identity of the endothelial L-selectin ligand involved during acute inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Bruce Furie for the PSGL-1−/− mice, Dr Shin-ichi Sato for the L-selectin−/− mice, Dr Reiji Kannagi for FucT-IV−/−/VII−/− mice, and Dr Barry Wolitzky for the 9A9 mAb. We also thank Ms Yuko Furukawa for technical assistance and Drs Klaus Ley and Toshiyuki Tanaka for helpful suggestions.
This work was supported by a grant-in-aid for the 21st Century Center of Excellence Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan; a grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science, Japan; a grant of Long-range Research Initiative (LRI) by Japan Chemical Industry Association (JCIA); and National Institutes of Health grants CA105001, CA96547, and AI56363.
National Institutes of Health
Authorship
Contribution: A.S. and M.Ma. designed and performed research; T.F.T. and J.B.L. contributed vital new reagents and analyzed data; M.Mi. designed research and analyzed data; and T.H. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takako Hirata, Laboratory of Combined Research on Microbiology and Immunology, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail: thirata@biken.osaka-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal