Abstract
UV-C irradiation has been shown to be effective for pathogen reduction in platelet concentrates, but preliminary work indicated that UV-C irradiation of platelets can induce platelet aggregation. In this study, the mechanism underlying this phenomenon was investigated. Irradiation of platelets with UV-C light (1500 J/m2) caused platelet aggregation, which was dependent on integrin αIIbβ3 activation (GPIIb/IIIa). This activation occurred despite treatment with several signal transduction inhibitors known to block platelet activation. UV-C also induced activation of recombinant αIIbβ3 in Chinese hamster ovary (CHO) cells, an environment in which physiologic agonists fail to activate. Activation of αIIbβ3 requires talin binding to the β3 tail, yet αIIbβ3-Δ724 (lacking the talin binding site) was activated by UV-C irradiation, excluding a requirement for talin binding. The UV-C effect appears to be general in that β1 and β2 integrins are also activated by UV-C. To explain these findings, we investigated the possibility of UV-C–induced photolysis of disulfide bonds, in analogy with the activating effect of reducing agents on integrins. Indeed, UV-C induced a marked increase in free thiol groups in platelet surface proteins including αIIbβ3. Thus, UV-C appears to activate αIIbβ3 not by affecting intracellular signal transduction, but by reduction of disulfide bonds regulating integrin conformation.
Introduction
Viral and, especially, bacterial contamination of platelet concentrates remains an issue for platelet transfusions.1 To minimize contamination of blood platelets, several pathogen reduction approaches have been developed that rely on irradiation with ultraviolet light (UV) in combination with a photosensitizer.2-4 Recently, the possibility of using UV-C light without the addition of an exogenous sensitizer has been explored.5,6 This approach uses UV-C at a wavelength of 254 nm, which is highly absorbed by nucleic acids, resulting in cyclobutane pyrimidine dimer formation and DNA degradation.7,8 Since no photosensitizer needs to be added to the platelet concentrate, UV-C–based pathogen inactivation should be easier to implement in existing blood bank procedures
UV-based pathogen reduction in blood platelets has a few drawbacks, as some properties of platelets are affected by UV irradiation. Van Marwijk and colleagues observed that UV-B irradiation resulted in increased fibrinogen binding to platelets.9 Furthermore, the UV-B–induced aggregation appeared to be dependent on PKC activation, signifying an important role for platelet signaling in UV-B–mediated activation of integrin αIIbβ3, the receptor binding fibrinogen.
As a member of the integrin family, αIIbβ3 consists of a large type I transmembrane α/β heterodimer, which is capable of bidirectional signaling through the plasma membrane. On unstimulated platelets, αIIbβ3 resides in an inactive conformation on the plasma membrane, but it is rapidly switched to an “on” state when the platelet becomes activated after stimulation with agonists such as thrombin, collagen, or adenosine diphosphate (ADP). With the αIIbβ3-activating properties of UV-B in mind, this study was performed to investigate whether UV-C irradiation induces similar changes in platelets. Our study, however, provides evidence that agonist-induced platelet responses that normally lead to αIIbβ3 activation do not play a role in UV-C–mediated αIIbβ3 activation. Instead, UV-C irradiation exerts a direct effect on αIIbβ3 (and other integrins) by modifying extracellular disulfide bonds regulating integrin conformation.
Methods
Materials
The monoclonal antibody PAC-1 binding to activated αIIbβ3 (conjugated to fluorescein isothiocyanate [FITC]) and the anti-β3 antibody (clone 1) used for immunoblotting were purchased from BD Biosciences (San Jose, CA). A control experiment with platelets from a Glanzmann patient lacking expression of αIIbβ3 proved the specificity of the β3 antibody, since the immunoreactive band (running above 95 kDa under reduced conditions) was absent in this sample. FITC-labeled antihuman fibrinogen antibody was obtained from WAK-Chemie Medical GmbH (Steinbach, Germany). The adenylate cyclase stimulator forskolin; the PKC inhibitors Ro 31-8220, Rottlerin, and staurosporin; the PI3-kinase inhibitor wortmannin; and streptavidin-coated agarose beads were obtained from Sigma (Zwijndrecht, The Netherlands). The PKC inhibitor Ly333531 was purchased from AG Scientific (San Diego, CA). The intracellular Ca2+ chelator BAPTA/AM was obtained from Molecular Probes Europe (Leiden, The Netherlands). Monoclonal antibody directed against CD61 (β3) or isotype-matched control IgG1, both labeled with FITC, were purchased from Sanquin (Amsterdam, The Netherlands). Goat anti–mouse IgG labeled with IRDye 800CW was obtained from LI-COR Biosciences (Lincoln, NE). Protease inhibitor mix was purchased from Roche (Basel, Switzerland). Tirofiban (Aggrastat) was obtained from Merck (Whitehouse Station, NJ). Calcein and maleimide conjugated to Alexa-633 were purchased from Molecular Probes Europe. Maleimide conjugated to biotin (1-biotinamido-4-[4′-maleimido-methyl)cyclohexanecarboxamido] butane, BMCC) was obtained from Pierce Biotechnology (Rockford, IL).
Preparation of platelet concentrates
Platelet concentrates (PCs) were prepared from whole blood–derived buffy coats essentially as previously described6,10 except that buffy coats were not pooled and Composol-PS (Fresenius HemoCare, Emmer Compascuum, The Netherlands) was added as synthetic storage medium (lowering the residual plasma to 30%). The platelet concentrates were kept at a constant temperature of 22°C in a platelet incubator (Helmer PF96, Noblesville, IN) for 1 or 2 days before use.
In most experiments, plasma was removed by washing the platelets in HEPES buffer (132 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1.2 mM K2HPO4, 20 mM HEPES, pH 7.4) with acid citric dextrose (ACD; 85 mM trisodium citrate, 71 mM citric acid, and 111 mM dextrose) added at one-tenth of the total volume. After another wash in HEPES buffer containing 5 mM glucose, the platelets were resuspended at 50 × 106 cells/mL in HEPES buffer containing 5 mM glucose and 1 mM CaCl2. Platelets were allowed to rest for 30 minutes at 37°C before use. In some experiments, washed platelets at a concentration of 1000 × 106 cells/mL were treated with digitonin (150 μM) for 1 minute at room temperature. The platelet “ghosts” were then washed 2 times with HEPES buffer and resuspended to a concentration of 50 × 106 cell equivalents/mL in HEPES buffer (containing glucose and CaCl2).
Isolation of other cells expressing integrins
The αIIbβ3 and αIIbβ3-Δ724 expressing chinese hamster ovary (CHO) cell lines have been described before.11 Human neutrophils were isolated by Percoll gradient centrifugation12 and the human promyelocytic leukemia cell line HL-60 was cultured at a cell density of 1 to 2 × 106 cells/mL in Iscove modified Dulbecco medium (IMDM; BioWhittaker, Brussels, Belgium) supplemented with 10% fetal calf serum (Gibco), 2 mM glutamine, penicillin (125 U/mL), and streptomycin (125 μg/mL) at 37°C in a humidified incubator with 5% CO2.
UV-C irradiation
All UV-C irradiations were performed with a suspension depth of 1 mm, except in the adhesion experiments with HL-60 cells and neutrophils, which had a suspension depth of approximately 5 mm. Unless indicated otherwise, 250 μL platelet suspension at 5 × 107 cells/mL, or CHO cells at 1 to 5 × 106 cells/mL, were added to a 24-well cell-culture plate (Nunc Maxisorp, Wiesbaden, Germany). In most experiments, the plate (without cover) was then irradiated from above with a low-pressure mercury arc lamp (emission line at 254 nm; Germicidal 15T/8-General Electric, Fairfield, CT) with constant intensity (0.5 mW/cm2) for 300 seconds at room temperature under continuous shaking, resulting in a dose of 1500 J/m2 UV-C. In some experiments, the light intensity was varied by varying the distance between the UV-C lamp and the incubation plate. The dose of UV-C light delivered was measured with a photo radiometer with UV sensor (Model UVX; UVP, Upland, CA).
PAC-1 binding assay
Platelets at a concentration of 5 × 107 cells/mL, or CHO cells at 2 × 106 cells/mL, were incubated with 40 μg/mL PAC-1 FITC or 10 μg/mL CD61-FITC (clone C17) or control IgG-FITC for 20 minutes at room temperature. The platelet suspensions were then diluted 10-fold, or diluted 3-fold for the CHO cells, with PBS containing 0.5% formaldehyde. Green fluorescence (FL1) was subsequently measured with a FACSCAN flow cytometer (BD, Franklin Lakes, NJ). To correct for varying αIIbβ3 expression in CHO cells, PAC-1 fluorescence (corrected for PAC-1 binding to untreated cells) was divided by the mean fluorescence intensity (MFI) values observed with CD61 antibody (C17). All MFI values were first corrected for isotype-matched control IgG binding.
Adhesion measurements
HL-60 cells or neutrophils, resuspended in HEPES buffer (containing glucose and CaCl2) at a concentration of 5 × 106/mL, were labeled with 4 μg/mL calcein for 30 minutes at 37°C. After 2 washing steps, labeled cells were resuspended in HEPES buffer (containing glucose and CaCl2) medium at a concentration of 2 × 106/mL. Cell adhesion was determined in 96-well Maxisorp plates (Nunc, Wiesbaden, Germany), uncoated or precoated with 10 μg/mL human plasma–derived fibronectin (Sigma) for 16 hours at 4°C. Calcein-labeled cells (100 μL) were pipetted in the 96-well plate and stimulated with 100 ng/mL PMA or irradiated with 1500 J/m2 UV-C in the presence or absence of MoAbs HP2/1 (anti-CD49d), SAM-1(anti-CD49e; Sanquin), or MoAb 44a (anti-CD11b; ATCC, Rockville, MD). Plates were then incubated for 30 minutes at 37°C and after 3 gentle washing steps with PBS, adherent cells were lysed with 100 μL 0.5% (wt/vol) Triton TX-100. Fluorescence was measured with a Spectrafluor Plus platereader (Tecan, Männedorf, Switzerland) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. Adhesion was determined as a percentage of total cell input measured in parallel.
Detection of free thiol groups
Washed platelets at a concentration of 300 × 106 cells/mL were left untreated, irradiated with 1500 J/m2 UV-C, or incubated for 10 minutes at room temperature (RT) with 10 mM dithiothreitol (DTT). The DTT-treated platelets were washed 3 times to remove interfering DTT. To measure the total amount of free thiol groups on the platelet surface, 50 × 106 platelets/mL were incubated for 20 minutes at room temperature with 0.2 μg/mL maleimide conjugated to Alexa633. After a 10-fold dilution in HEPES buffer, binding fluorescence was measured on an LSRII flow cytometer (BD).
To measure free thiol groups in αIIbβ3, 300 × 106 platelets/mL were incubated for 10 minutes at room temperature with 10 μM BMCC. After a washing step to remove unbound BMCC, the platelet suspension was lysed with 3× concentrated RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 10 mM Tris, pH 7.4, containing protease inhibitor mix). Then, the platelet lysates were incubated with streptavidin-coated agarose beads for 2 hours at 4°C. Afterward, the beads were washed 3 times with RIPA buffer, resuspended in 50 μL Laemmli sample buffer containing 50 mM DTT, and heated for 10 minutes at 95°C before storage at −20°C. Before sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretic transfer to nitrocellulose membranes, the samples were again heated to 95°C for 5 minutes. After transfer, the blots were blocked with 5% (wt/vol) nonfat dry milk in TBST (150 mM NaCl, 10 mM Tris/HCl, 0.05% Tween-80 [wt/vol]) for 1 hour at room temperature and subsequently incubated with a monoclonal antibody specific for the β3 integrin (diluted in 2.5% nonfat dry milk in TBST) for 16 hours at 4°C. After washing 3 times in TBST, detection of β3 chains in the precipitates (and in the total lysates) was completed by incubation with goat anti–mouse IgG IRDye 800CW and quantification of bound antibodies (after extensive washing of the blots in PBS) on an Odyssey Infrared Imaging system (LI-COR Biosciences, Lincoln, NE).
Results and discussion
UV-C dose-dependently increases activation of αIIbβ3 on platelets
Initial experiments with UV-C–irradiated platelets showed a lowered platelet count after several days of storage, possibly caused by aggregation. When platelets were irradiated with somewhat higher doses of UV-C (1500 J/m2), the platelet count decreased immediately after irradiation (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), and large aggregrates were detectable under the light microscope (Figure S1B). Because tirofiban, a synthetic αIIbβ3 antagonist, completely prevented both phenomena (Figure S1), these observations clearly pointed toward an UV-C–induced aggregation as a consequence of αIIbβ3 activation.
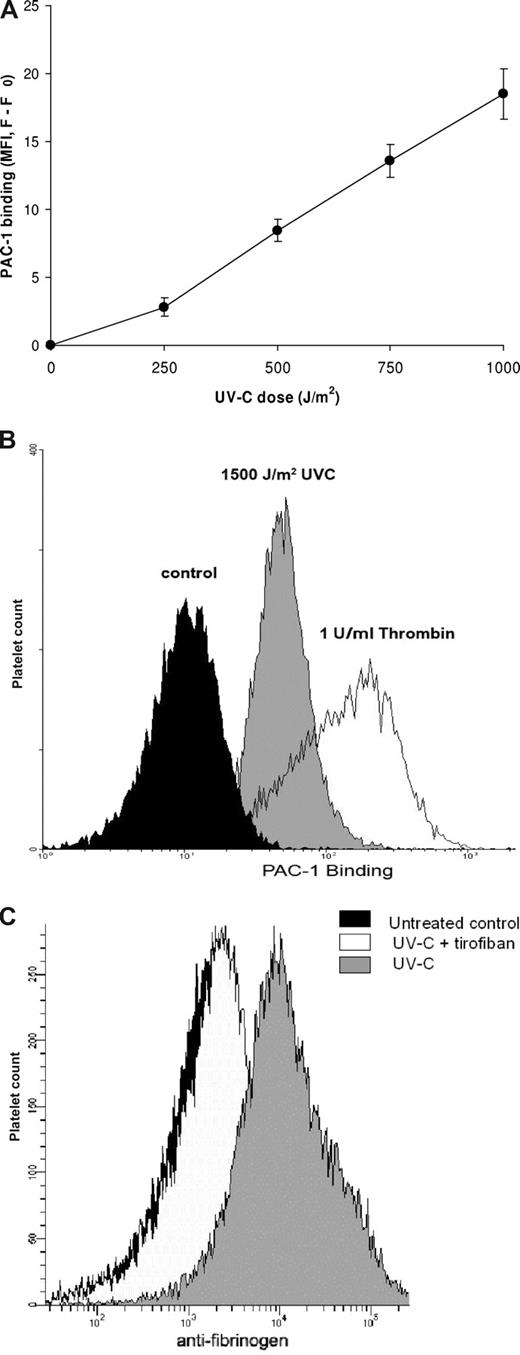
A useful tool for detecting activated αIIbβ3 is MoAb PAC-1, which selectively recognizes the high-affinity conformation of αIIbβ3.13 When washed platelets were exposed to increasing dosages of UV-C, binding of PAC-1 increased in a dose-dependent fashion (Figure 1A). The extent of PAC-1 binding was lower than that obtained with thrombin stimulation, even with a dose of 1500 J/m2 as used in most experiments (Figure 1B). This difference might be related to the observation that UV-C irradiation, in contrast to thrombin stimulation, did not induce a significant up-regulation of αIIbβ3 from intracellular granules (data not shown). Since addition of apyrase to counter the effect of ADP release also did not affect the UV-C effect on PAC-1 binding (data not shown), granule secretion seemed not to be involved in UV-C–induced αIIbβ3 activation. With 2% plasma added as source of fibrinogen, UV-C induced increased binding of fibrinogen to the platelets (Figure 1C), confirming activation of αIIbβ3.
UV-C irradiation of platelets activates αIIbβ3. (A) An increasing dose of UV-C was delivered to platelets by decreasing the distance between lamp and incubation plate and thereby increasing the light intensity, while irradiation time was kept constant. Higher doses of UV-C caused increased PAC-1 binding to the platelets, resulting in higher fluorescence. Data were corrected for PAC-1 binding to unstimulated platelets and are represented as the mean plus or minus SEM (n = 6). (B) Histograms depicting higher PAC-1 binding after UV-C irradiation (shaded histogram) or thrombin stimulation (open histogram) as compared with the untreated control (filled histogram). This experiment is representative of 3 similar experiments. (C) Histograms depicting higher binding of antifibrinogen antibody (as detected by goat anti–mouse IgG-FITC) after UV-C irradiation (open histogram) as compared with the untreated control (filled histogram). The increase in binding induced by UV-C was prevented by inclusion of tirofiban (1 μg/mL, shaded histogram). This experiment is representative of 3 similar experiments.
UV-C irradiation of platelets activates αIIbβ3. (A) An increasing dose of UV-C was delivered to platelets by decreasing the distance between lamp and incubation plate and thereby increasing the light intensity, while irradiation time was kept constant. Higher doses of UV-C caused increased PAC-1 binding to the platelets, resulting in higher fluorescence. Data were corrected for PAC-1 binding to unstimulated platelets and are represented as the mean plus or minus SEM (n = 6). (B) Histograms depicting higher PAC-1 binding after UV-C irradiation (shaded histogram) or thrombin stimulation (open histogram) as compared with the untreated control (filled histogram). This experiment is representative of 3 similar experiments. (C) Histograms depicting higher binding of antifibrinogen antibody (as detected by goat anti–mouse IgG-FITC) after UV-C irradiation (open histogram) as compared with the untreated control (filled histogram). The increase in binding induced by UV-C was prevented by inclusion of tirofiban (1 μg/mL, shaded histogram). This experiment is representative of 3 similar experiments.
UV-C activation of platelet αIIbβ3 does not require physiologic signal transduction pathways
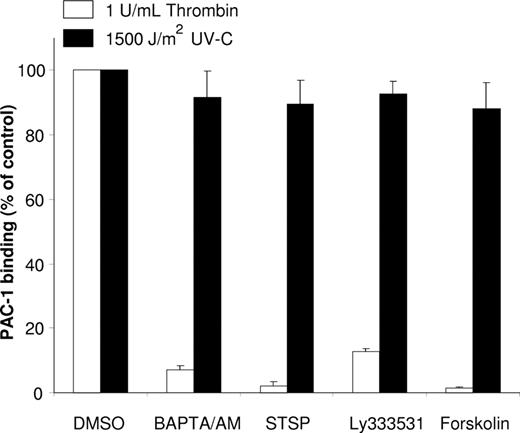
It has been described by van Marwijk and colleagues9 that UV-B irradiation activates αIIbβ3 via a PKC-dependent pathway. We observed that, upon UV-C irradiation of platelets, PAC-1 binding occurred in the presence of several inhibitors of agonist-induced αIIbβ3 activation (Figure 2). In addition, digitonin permeabilization of platelets14 to induce leakage of cytosolic proteins completely abrogated the response to PMA and thrombin, but had no effect on the UV-C–induced αIIbβ3 activation (Figure S2). When we measured in intact platelets activation of the small GTPase RAP1b, a key player in agonist-induced integrin activation in haemopoetic cells,15-17 thrombin stimulation induced a clear effect, whereas, again, UV-C did not exert an effect (data not shown).
αIIbβ3 activation by UV-C irradiation is not dependent on intracellular signaling. Platelets were preincubated for 30 minutes at 37°C with various inhibitors of platelet activation: 30 μM BAPTA/AM, 1 μM staurosporin (STSP), 10 μM Ly333531, or 20 μM forskolin and subsequently stimulated at 37°C for 5 minutes with 1 U/mL thrombin (□) or irradiated with 1500 J/m2 UV-C (■) as described in “Methods.” The PAC-1 binding to the controls (with DMSO added) was normalized to 100%, which had a mean fluorescence intensity (MFI; Fpac1 − F0) of 162 (±24) for the thrombin-stimulated platelets and an MFI of 44 (±10) for the UV-C–irradiated platelets. The mean fluorescence of PAC-1 to unstimulated platelets was used for background subtraction (F0). Data represent the mean plus or minus SD of 3 experiments.
αIIbβ3 activation by UV-C irradiation is not dependent on intracellular signaling. Platelets were preincubated for 30 minutes at 37°C with various inhibitors of platelet activation: 30 μM BAPTA/AM, 1 μM staurosporin (STSP), 10 μM Ly333531, or 20 μM forskolin and subsequently stimulated at 37°C for 5 minutes with 1 U/mL thrombin (□) or irradiated with 1500 J/m2 UV-C (■) as described in “Methods.” The PAC-1 binding to the controls (with DMSO added) was normalized to 100%, which had a mean fluorescence intensity (MFI; Fpac1 − F0) of 162 (±24) for the thrombin-stimulated platelets and an MFI of 44 (±10) for the UV-C–irradiated platelets. The mean fluorescence of PAC-1 to unstimulated platelets was used for background subtraction (F0). Data represent the mean plus or minus SD of 3 experiments.
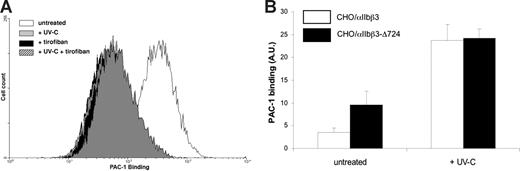
These results supported the notion that, in contrast to the effect induced by UV-B irradiation,9 intracellular signal transduction was not involved in the UV-C–induced αIIbβ3 activation. However, these results did not exclude a possible role for the cytoskeletal protein talin. Talin binds to the cytoplasmic tail of β3 and activates αIIbβ3 via inside-out signaling.18 It was conceivable that UV-C irradiation could induce talin binding to αIIbβ3 via an unknown mechanism. To investigate this possibility, A5 cells (CHO cells expressing wild-type αIIbβ3) and CHO cells expressing αIIbβ3-Δ724, a talin binding deficient truncation mutant,11 were irradiated with UV-C. Because the expression of αIIbβ3-Δ724 was much less than αIIbβ3, PAC-1 binding was corrected for this difference in integrin expression. UV-C irradiation increased PAC-1 binding to the cells expressing wild-type αIIbβ3 (Figure 3A) and to the cells expressing the αIIbβ3-Δ724 mutant (Figure 3B). The first result corroborated our results in digitonin-permeabilized platelets, because A5 cells lack a functional pathway to αIIbβ3 activation, making them insensitive toward stimulation with various agonists including PMA.16 The activation observed in CHO cells expressing the αIIbβ3-Δ724 mutant excluded a role for talin binding in UV-C–induced αIIbβ3 activation.
UV-C activates αIIbβ3 and αIIbβ3-Δ724 in CHO cells. (A) CHO cells expressing αIIbβ3 show increased PAC-1 binding after UV-C irradiation. This binding is specific for αIIbβ3, as the antagonist tirofiban (1 μg/mL) inhibited PAC-1 binding in untreated cells and UV-C–irradiated cells. This graph is representative of 5 similar experiments. (B) CHO A5 cells (□) or CHO αIIbβ3-Δ724 cells (■) were irradiated with 1500 J/m2 UV-C. Activation of αIIbβ3 was measured with PAC-1 and was expressed as percentage of C17-FITC binding to correct for differences in αIIbβ3 expression. Binding of FITC-conjugated C17 to CHO/αIIbβ3 resulted in an MFI (FC17 − FIgG) value of 1165 (±165), and with CHO/αIIbβ3-Δ724 cells this value was 277 (± 91). Data represent the mean plus or minus SEM of 5 experiments.
UV-C activates αIIbβ3 and αIIbβ3-Δ724 in CHO cells. (A) CHO cells expressing αIIbβ3 show increased PAC-1 binding after UV-C irradiation. This binding is specific for αIIbβ3, as the antagonist tirofiban (1 μg/mL) inhibited PAC-1 binding in untreated cells and UV-C–irradiated cells. This graph is representative of 5 similar experiments. (B) CHO A5 cells (□) or CHO αIIbβ3-Δ724 cells (■) were irradiated with 1500 J/m2 UV-C. Activation of αIIbβ3 was measured with PAC-1 and was expressed as percentage of C17-FITC binding to correct for differences in αIIbβ3 expression. Binding of FITC-conjugated C17 to CHO/αIIbβ3 resulted in an MFI (FC17 − FIgG) value of 1165 (±165), and with CHO/αIIbβ3-Δ724 cells this value was 277 (± 91). Data represent the mean plus or minus SEM of 5 experiments.
UV-C also activates β2 and β1 integrins
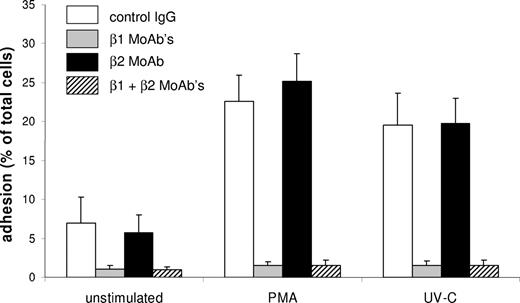
Given the similarities in the activation of different integrins, we next examined the effect of UV-C irradiation on β1 and β2 members of the integrin family. HL-60 cells have a relatively low surface expression of β2 integrins and mainly use β1 integrins α4β1 (VLA4) and α5β1 (VLA5) for adhesion to fibronectin.19 Both PMA stimulation and UV-C irradiation resulted in increased adhesion of HL-60 cells to Fn-coated wells (Figure 4). UV-C–induced adhesion was blocked by antibodies against α4β1 and α5β1 (MoAbs HP2/1 and SAM-1) but not by PKC or PI3-K inhibitors (data not shown), indicating that integrin activation was not triggered via PKC or PI3-K. Similar results were obtained with human neutrophils expressing the integrin αmβ2 (CD11b/CD18). In these cells, UV-C induced adherence to uncoated plastic, which was sensitive to the αmβ2-blocking MoAb 44a, but not to PKC and PI3-K inhibitors (data not shown).
UV-C irradiation induces β1 integrin–mediated adhesion of HL-60 cells to Fn. HL-60 cells were incubated in wells coated with Fn and stimulated with PMA or irradiated with UV-C in the presence of different antibodies. Addition of control IgG (□) did not inhibit adhesion. Blocking of αmβ2 with MoAb 44a (■) also had no effect on adhesion. Blocking of α4β1 and α5β1 with MoAbs HP2/1 and SAM-1 ( ) completely prevented adhesion to Fn. Data represent the mean plus or minus SD of 3 experiments.
) completely prevented adhesion to Fn. Data represent the mean plus or minus SD of 3 experiments.
UV-C irradiation induces β1 integrin–mediated adhesion of HL-60 cells to Fn. HL-60 cells were incubated in wells coated with Fn and stimulated with PMA or irradiated with UV-C in the presence of different antibodies. Addition of control IgG (□) did not inhibit adhesion. Blocking of αmβ2 with MoAb 44a (■) also had no effect on adhesion. Blocking of α4β1 and α5β1 with MoAbs HP2/1 and SAM-1 ( ) completely prevented adhesion to Fn. Data represent the mean plus or minus SD of 3 experiments.
) completely prevented adhesion to Fn. Data represent the mean plus or minus SD of 3 experiments.
UV-C light induces photolysis of disulfide bonds in αIIbβ3
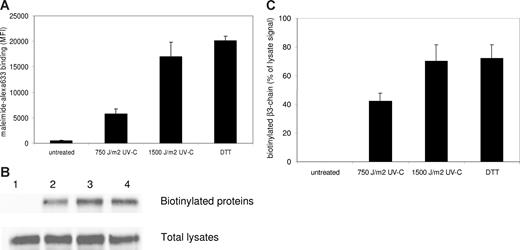
The ability of UV-C to activate several members of the integrin family independent of cellular signaling suggested a direct chemical modification as the cause of integrin activation. It has recently been reported that UV-C is able to break disulfide bonds in plasma proteins via photolysis.20 Moreover, reduction of disulfide bonds by DTT treatment is known to activate several members of the integrin family.21,22 We therefore investigated whether UV-C irradiation induced the reduction of disulfide bonds in αIIbβ3. UV-C irradiation did, indeed, cause a great increase in free thiol groups on the surface of human platelets, as measured by a fluorescent probe coupled to maleimide (Figure 5A). To investigate the effect on αIIbβ3 specifically, free thiol groups were labeled with biotin after the various treatments used in Figure 5A, and biotinylated proteins were then precipitated with streptavidin-agarose. Western blots of the total lysates incubated with streptavidin-HRP indicated no major differences in overall biotinylation (data not shown). However, as shown in Figure 5B, only after UV-C irradiation αIIbβ3 appeared to be labeled with biotin, indicating a strong increase in free thiol groups by photolysis of disulfide bonds in αIIbβ3. This increase showed a similar dose dependence (Figure 5C) as the increase in total free thiol groups on the membrane surface (Figure 5A).
UV-C irradiation induces disulfide bridge reduction in αIIbβ3. (A) Platelets were either left untreated, irradiated with 2 doses of UV-C light, or treated with DTT, and then incubated with maleimide coupled to the fluorescent dye alexa633 to label free thiol groups. Binding of the probe was assessed by flow cytometry. Data represent the mean plus or minus SEM of 4 experiments. (B) After the treatments described under panel A, designated 1-4, platelets were incubated with BMCC to biotinylate free thiol groups. Biotinylated proteins were then precipitated with streptavidin-agarose beads. The top row shows a Western blot of the precipitates (after incubation with a MoAb specific for β3) to detect biotinylated β3 chains, the bottom row shows a Western blot with the same antibody of the total lysates. The blots shown are representative for 3 experiments. (C) Bar graph depicting the binding of the β3 antibody to the immunoblots of the precipitates as quantified on a Odyssey Infrared Imaging system and calculated as percentage of total β3 present in the lysates to correct for input differences. Data represent the mean plus or minus SEM of 3 experiments.
UV-C irradiation induces disulfide bridge reduction in αIIbβ3. (A) Platelets were either left untreated, irradiated with 2 doses of UV-C light, or treated with DTT, and then incubated with maleimide coupled to the fluorescent dye alexa633 to label free thiol groups. Binding of the probe was assessed by flow cytometry. Data represent the mean plus or minus SEM of 4 experiments. (B) After the treatments described under panel A, designated 1-4, platelets were incubated with BMCC to biotinylate free thiol groups. Biotinylated proteins were then precipitated with streptavidin-agarose beads. The top row shows a Western blot of the precipitates (after incubation with a MoAb specific for β3) to detect biotinylated β3 chains, the bottom row shows a Western blot with the same antibody of the total lysates. The blots shown are representative for 3 experiments. (C) Bar graph depicting the binding of the β3 antibody to the immunoblots of the precipitates as quantified on a Odyssey Infrared Imaging system and calculated as percentage of total β3 present in the lysates to correct for input differences. Data represent the mean plus or minus SEM of 3 experiments.
In conclusion, although UV-C irradiation is a potentially superior means to reduce bacteria in platelet concentrates, its application may be limited by the activation of the integrin αIIbβ3 reported here. It should be noted that the dose used in this study is significantly higher than required for inactivation of most pathogens5,6 and thus one can expect the activation of only a minor part of total αIIbβ3 present. However, during storage further platelet activation can take place due to outside-in signaling after binding of fibrinogen present in the plasma. Thus, this study identifies an unexpected consequence of UV-C irradiation of transfusion products and suggests that future studies, directed at means of preventing it, seem warranted.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr A. Tool for his help with the adhesion experiments.
This work was supported by grant HL 078784 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: R.V. performed research and wrote the first draft of the manuscript; D.W.C.D. and I.M.DeC. performed experiments and analyzed data; M.H.G. contributed essential reagents and suggested key experiments; and D.deK. and A.J.V. designed the study and supervised writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arthur J. Verhoeven, Department of Blood Cell Research, Sanquin Research, 1066 CX Amsterdam, The Netherlands; e-mail: a.verhoeven@sanquin.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal