Abstract
We demonstrate that the secretome of mesenchymal stromal cells (MSCs) suppresses plasma cell (PC) immunoglobulin (Ig) production, induces plasmablast proliferation, and leads to interleukin-10–mediated blockade in vitro. We found that these effects are the result of MSC-derived CC chemokine ligands CCL2 and CCL7. More specifically, MSCs further processed these CC chemokines by the activity of matrix metalloproteinases (MMPs), leading to the generation of proteolytically processed antagonistic CCL2 variant. Neutralizing CCL2 or inhibiting MMP enzymatic activity abolished the PC-suppressive effect of MSCs. We also observed that MMP-processed CCL2 suppresses signal transducer and activator of transcription 3 (STAT3) activation in PC. As a result, the transcription factor PAX5 is induced, thus explaining the inhibition of Ig synthesis. The absence of inhibitory effects by MSC on the humoral response of CCR2−/− mice to xenoantigen suggests that MMP-cleaved CCL2/CCR2 interaction as well as downstream phosphatase activity is necessary for antagonistic effect. We tested syngeneic MSCs in hemophilic B6 mice with predeveloped antihuman factor VIII (hFVIII) antibodies and demonstrated a robust decrease in hFVIII-specific IgG levels. Thus, MSCs may play a role in modulating Ig production by PCs via MMP processing of CCL2 and may represent an appealing cell therapy approach for pathologic humoral responses.
Introduction
Mesenchymal stromal cells (MSCs) normally reside in marrow where they lay as pericytes and interstitial cells. MSCs have long been thought to play a supportive role in hematopoiesis and lymphopoiesis as part of the marrow microenvironmental niche and have been the object of more than quarter century of research as bone mesenchymal precursors.1 The precise interaction between MSCs and hematopoietic cells remains to be determined but appears to involve complex contact and paracrine factors, which can be recapitulated in vitro by the stroma-dependent long-term bone marrow culture hematopoietic assay.2 More recently, it was discovered that MSCs possessed potent immune-modulatory properties, both suppressive and stimulatory, which could be exploited clinically.3-5 Indeed, MSCs can be harvested from a simple low-volume aspirate, ex vivo–expanded to large numbers with routine tissue-culture techniques, and subsequently readministered to either autologous or allogeneic recipients with clinical effects.5 The use of ex vivo–expanded MSCs in suppressing T-cell reactivity via secreted soluble factors opened a new field of interest in cellular immunosuppression,3,6 well exemplified by the remarkable effects of allogeneic MSCs in attenuating the symptoms of steroid-resistant graft-versus-host disease (GVHD).7 The immune-suppressive effects of MSCs also may extend to pathologic autoreactive lymphocytes, at least in animal models of autoimmune disease. Indeed, experimental autoimmune encephalomyelitis was strikingly ameliorated in mice after injection of MSCs,8 and such preclinical results buttress the use of MSCs as a cell-based pharmaceutical in more than 2 dozen clinical trials worldwide for this and other autoimmune conditions and regenerative medicine applications (www.clinicaltrials.gov). However, the molecular mechanisms by which MSCs mediate their suppressive effect in vivo remain controversial. Most data generated to date with human MSCs were inferred from in vitro experimentation, which has identified multiple, at times conflicting, candidate pathways.5
MSCs have also been shown to suppress B cell–dependent immunoglobulin (Ig) production in vitro as well by unknown mechanisms.9 Although it is thought that marrow resident MSCs are linked to lymphoid development, the underpinning molecular mechanism by which this relationship and modulatory effects occur are unknown. Recent in vivo studies performed in mice with experimental GVHD identified a key role to MSC interferon-gamma responsiveness and their ability to generate nitric oxide (NO) and speculated that supplementary components of the MSC secretome, including chemokines, were at play.10 Studies performed on MSC secretome revealed the presence of a wide range of cytokines and chemokines, including CC chemokine ligand 2 (CCL2).10 Furthermore, MSCs are also capable of producing an array of matrix metalloproteinase (MMPs) with the capacity of cleaving various target molecules, including chemokines.11-13 Such MMP processing, at least on CC chemokines, was demonstrated to convert the biochemical property of the targeted molecules from an agonist to an antagonist form.13 Knowing that B-cell development is dependent in part on the close interaction of B-cell progenitors with MSCs and that unidentified soluble factors produced by MSCs can also suppress B-cell activation, proliferation, and differentiation to Ig-producing cells in vitro,9 we hypothesize that MSCs may modulate Ig production by plasma cells (PCs) as part of a physiologic response to xenoantigen or in the setting of pathologic humoral responses. More specifically, we investigated the capacity of MSCs in modulating Ig production and PC biology both in vitro and in vivo by specifically studying CCL2 and its MMP-processed derivative that can target CCR2-expressing PCs. The observations made here could give insights on the physiologic role of MSCs in marrow PC homeostasis as well as in their properties when administered as an anti-inflammatory biopharmaceutical.
Methods
Mice and immunizations
Wild-type (WT) and CCR2−/− B6 mice were all age-matched and purchased from The Jackson Laboratory (Bar Harbor, ME). Hemophilic B6 mice were kindly provided by Dr Mark Blostein (Lady Davis Institute, Montreal, QC). TC-PTP−/− mice were kindly provided by Dr Michel Tremblay (McGill University, Montreal, QC). Recombinant ovalbumin (rOVA) immunizations were performed intraperitoneally at a concentration of 10 μg per animal twice at an interval of 2 weeks. For recombinant human (rh) FVIII experiments, 0.02 U/g weight was delivered 4 times every 2 days for the first week followed by a single injection 1 week later. A total of 2 million syngeneic or allogeneic MSCs are injected intraperitoneally at the same sites of rOVA immunizations once high IgG levels are reached. Approval of animal studies was obtained from the Lady Davis Institute Animal Care Committee.
Proteins, chemicals, antibodies, and cytokine ELISA
Reagents such as rOVA, rhMMP1, ascorbic acid-2 phosphate, insulin, Alizarin Red S, Oil Red O, and actinonin were purchased from Sigma Diagnostics Canada (Mississauga, ON). Antimouse IgG secondary antibodies used in enzyme-linked immunospot (ELISPOT) assays were purchased from GE Healthcare (Little Chalfont, United Kingdom). Anti-PAX5 and anti-pAKT/STAT3 were purchased from BD PharMingen (San Diego, CA) and Cell Signaling Technology (Danvers, MA), respectively. Anti-CCL2 antibody and all cytokine enzyme-linked immunosorbent assays (ELISAs) were purchased from R&D Systems (Minneapolis, MN) and used according to the manufacturer's instructions. Anti-MMP antibodies were purchased from Chemicon International (Temecula, CA).
Generation and differentiation of WT and CCL2−/−MSC
Whole bone marrow from femurs and tibias of a female B6 or BALB/c mice was harvested and placed in culture in complete media. Five days later, nonadherent cells were washed and adherent cells were kept in culture for a period of 5 to 6 weeks before the appearance of a homogeneous polyclonal population. For osteogenic differentiation, MSCs (70%-80% confluent) were cultured in media supplemented with β-glycerol phosphate (10 nM), dexamethasone (10−8 M), and ascorbic acid-2 phosphate (5 μg/mL) for 4 weeks with media changes every 2 to 3 days. Alizarin Red S was then used to stain calcium in the mineralized extracellular matrix as shown previously.14 To induce adipogenic differentiation, MSCs (50%-60% confluent) were cultured in complete media supplemented with indomethacin (46 μM), 3-isobutyl-methylxanthine (0.5 nM), dexamethasone (1 μM), and insulin (10 μg/mL) for 7 days with continual media changes every 2 days. Oil Red O was used for lipid droplet staining as reported previously.14
Flow cytometry
All antibodies used in flow cytometry were purchased from BD PharMingen. Antibody staining was performed according to the manufacturer's instructions.
ELISPOT assays, protein arrays, and RT-PCR
ELISPOTs were performed according to the manufacturer's instructions. Briefly, rOVA-coated ELISPOT plates (Millipore, Billerica, MA) were washed 3 times with phosphate-buffered saline (PBS), blocked with 1% bovine serum albumin (BSA), before the addition of test conditions. After 3 washes with PBS, secondary antimouse alkaline phosphatase-labeled antibodies were added for 4 hours at 4°C before development. Protein arrays (RayBiotech, Norcross, GA) were used to screen the secretome of MSCs according to the manufacturer's instructions. For CCR2 expression on MSCs, reverse-transcribed polymerase chain reaction (RT-PCR) was performed on RNA extracted using the AllPrep DNA/RNA Mini Kit (QIAGEN, Valencia, CA). The used primers were purchased from R&D Systems.
Generation of antagonist CCL2 in vitro
To generate antagonist CCL2 in vitro, 10 ng pure rhMMP1 was added directly to 50 μg pure rCCL2 for a period of 4 hours at 37°C. mpCCL2 was directly used in assays without further modifications.
Western analysis
For phosphorylated AKT, STAT3, and PAX5 analysis by Western blot, whole-cell lysate from sorted CD138+ cells was separated on 4% to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen Canada, Burlington, ON) and blotted with appropriate antibodies according to the manufacturer's instructions. For the detection of CCL2, conditioned media (CM) from MSCs or from CCL2−/− MSCs was collected and concentrated 20-fold using Amicon (Millipore) and then run on Tricine SDS-PAGE gel prepared as reported previously15 and probed with anti-CCL2 antibody. The same concentrated supernatant was run on 12% SDS-PAGE and probed with anti-MMP-1, MMP-3, and MMP-8.
CFSE labeling and in vitro proliferation
For in vitro proliferation assays, splenocytes were labeled with 5 μg/mL carboxyfluorescein diacetate succinimidyl ester (CFSE) for 8 to 10 minutes at 37°C and then washed once with CM and 3 times with PBS. To assess the proliferation over time, cells were stained with anti-CD138 daily and analyzed by flow cytometry.
Statistical analysis
P values were calculated by the paired Student t test.
Results
MSC-conditioned media inhibits Ig production by PCs
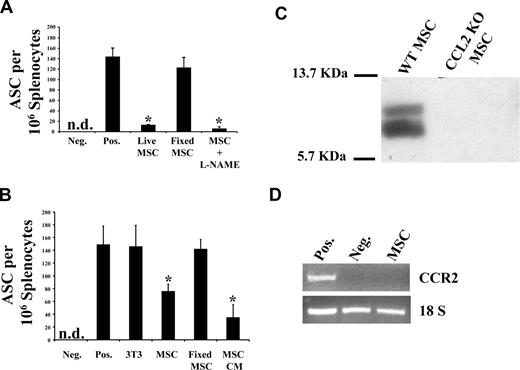
Splenocytes collected from B6 mice immunized with rOVA were treated in vitro with live or fixed MSCs and the number of antibody-secreting cells (ASCs) assessed by an OVA-specific ELISPOT assay (Figure 1A). The observed decrease in OVA-specific Ig-producing ASCs in the presence of MSCs is dependent on MSC-derived soluble factors because only metabolically active MSC or MSC-derived CM (Figure 1B) was inhibitory. This suggests the absence of a contact-dependent inhibitory mechanism on ASC-dependent Ig production. In addition, NO, a previously described MSC-produced T-cell inhibitor,16 does not seem to affect ASCs because inhibition of NO synthesis by the addition of the inhibitor L-NAME to MSC failed to rescue Ig secretion (Figure 1A). Several studies have suggested that leukocyte infiltration to inflammatory sites after CC chemokine secretion is regulated by enzymatic chemokine processing.13,17 Such regulation occurs through the proteolytic processing of CCL2/CCL7 at their N termini by MMPs converting these chemokines from agonists to antagonists of chemotaxis for the CCL2 receptor (CCR2)–expressing cells. Using protein arrays to identify candidate secreted molecules with potential inhibitory properties on Ig production, we identified the CC chemokine CCL2 whose secretion was confirmed by Western blot (Figure 1C). We also found that cultured MSCs do not express the mRNA encoding for CCR2 (Figure 1D).
MSC CM can block IgG secretion from PCs. (A) Live MSCs can block IgG secretion from splenocytes. Splenocytes collected from OVA-immunized mice were either cultured in vitro in splenocyte media or with MSC 24 hours before ELISPOT read-outs. For the L-NAME test group, MSCs were treated for 24 hours with the inhibitor before the addition of splenocytes; 4% paraformaldehyde (PFA) was used to fix MSC. Only metabolically active MSC, even in the presence of L-NAME, had IgG-inhibitory properties. Fixing MSC led to loss in inhibition (n = 3/group; P < .001). (B) MSCs secrete a soluble inhibitory factor(s). Based on the observation made previously, MSC CM was used alone in ELISPOT to demonstrate that it contains soluble factor(s) mediating IgG inhibition (n = 3/group; P < .001). (C) WT MSC led to MMP-cleaved CCL2. CM derived from WT MSC or CCL2−/− MSC was run on a Tricine SDS-PAGE to detect CCL2. (D) MSCs do not express CCR2. RNA extracted from MSCs was used in an RT-PCR to demonstrate the absence of CCR2 expression on MSCs, suggesting no autocrine loop induced by CCL2/CCR2 interaction.
MSC CM can block IgG secretion from PCs. (A) Live MSCs can block IgG secretion from splenocytes. Splenocytes collected from OVA-immunized mice were either cultured in vitro in splenocyte media or with MSC 24 hours before ELISPOT read-outs. For the L-NAME test group, MSCs were treated for 24 hours with the inhibitor before the addition of splenocytes; 4% paraformaldehyde (PFA) was used to fix MSC. Only metabolically active MSC, even in the presence of L-NAME, had IgG-inhibitory properties. Fixing MSC led to loss in inhibition (n = 3/group; P < .001). (B) MSCs secrete a soluble inhibitory factor(s). Based on the observation made previously, MSC CM was used alone in ELISPOT to demonstrate that it contains soluble factor(s) mediating IgG inhibition (n = 3/group; P < .001). (C) WT MSC led to MMP-cleaved CCL2. CM derived from WT MSC or CCL2−/− MSC was run on a Tricine SDS-PAGE to detect CCL2. (D) MSCs do not express CCR2. RNA extracted from MSCs was used in an RT-PCR to demonstrate the absence of CCR2 expression on MSCs, suggesting no autocrine loop induced by CCL2/CCR2 interaction.
Paracrine MSC processing of CCL2/CCL7 by MMPs
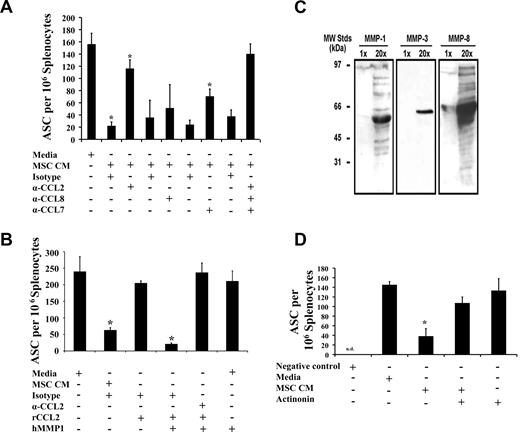
Ig production by ASCs was restored after CCL2 neutralization, whereas CCL7 inactivation had partial effects (Figure 2A). Taken together, our data suggest an additive inhibitory effect of mostly CCL2 and partly CCL7 on spleen-derived ASCs because their simultaneous neutralization completely rescued Ig secretion (Figure 2A). To determine the mechanism by which CCL2 inhibits Ig secretion, spleen-derived PCs were cultured in media with full-length recombinant CCL2 (rCCL2). The level of ASCs in that group was comparable with nontreated control (Figure 2B), whereas the addition of in vitro MMP1-processed rCCL2 (antagonist CCL2 as defined by others,13 hereafter mpCCL2) led to almost 90% Ig inhibition but could be reversed on the addition of CCL2-neutralizing antibody (Figure 2B). MSCs are known to secrete multiple MMPs, and Western blot analysis on MSC CM demonstrates the presence of MMP1, MMP3, and MMP8, all of which are implicated in CC chemokine processing and conversion to an antagonistic variant13 (Figure 2C). To further confirm the direct implication of MMPs in our model, CM derived from MSCs treated with actinonin, a broad MMP inhibitor, failed in suppressing ASCs (Figure 2D). These data suggest that MSCs secrete CCL2, which becomes targeted by MSC-derived MMPs generating an antagonist compound capable of blocking Ig production from ASCs.
MSC-derived MMP-processed CCL2/CCL7 block IgG secretion. (A) MSCs block IgG secretion via CCL2/7. An ELISPOT assay was performed in the presence of CCL2/8 and CCL7 neutralizing antibodies. IgG secretion is partially restored on CCL2 and CCL7 neutralization, but a complete rescue is achieved after simultaneous inhibition of both CCLs (n = 3/group; P < .001). (B) MSCs secrete antagonist CCL2. To prove that a truncated form of CCL2 with antagonistic activities is responsible for IgG blockade, in vitro MMP1-mpCCL2 was added on OVA-derived splenocytes and led to 90% IgG inhibition (n = 3/group; P < .001). (C) MSCs secrete MMP-1, MMP-3, and MMP-8. To generate the cleaved form of CCL2, an MMP digestion is required. MMPs were indeed detected in MSC CM by Western blot (WB). (D) MMP inhibition rescues IgG blockade. Because MMP enzymatic cleavage of CCL2 as well as CCL7 is responsible for the IgG secretion inhibition, we added actinonin (a generic MMP inhibitor) and demonstrate that PCs can secrete IgG after MSC CM treatment in a way comparable with the nontreated splenocytes (n = 3/group; P < .05). The negative control consists of splenocytes from naive mice. All experiments were repeated 3 times. Error bars represent plus or minus SD.
MSC-derived MMP-processed CCL2/CCL7 block IgG secretion. (A) MSCs block IgG secretion via CCL2/7. An ELISPOT assay was performed in the presence of CCL2/8 and CCL7 neutralizing antibodies. IgG secretion is partially restored on CCL2 and CCL7 neutralization, but a complete rescue is achieved after simultaneous inhibition of both CCLs (n = 3/group; P < .001). (B) MSCs secrete antagonist CCL2. To prove that a truncated form of CCL2 with antagonistic activities is responsible for IgG blockade, in vitro MMP1-mpCCL2 was added on OVA-derived splenocytes and led to 90% IgG inhibition (n = 3/group; P < .001). (C) MSCs secrete MMP-1, MMP-3, and MMP-8. To generate the cleaved form of CCL2, an MMP digestion is required. MMPs were indeed detected in MSC CM by Western blot (WB). (D) MMP inhibition rescues IgG blockade. Because MMP enzymatic cleavage of CCL2 as well as CCL7 is responsible for the IgG secretion inhibition, we added actinonin (a generic MMP inhibitor) and demonstrate that PCs can secrete IgG after MSC CM treatment in a way comparable with the nontreated splenocytes (n = 3/group; P < .05). The negative control consists of splenocytes from naive mice. All experiments were repeated 3 times. Error bars represent plus or minus SD.
MSC-derived CCL2 leads to plasmablast proliferation and interleukin-10–mediated blockade
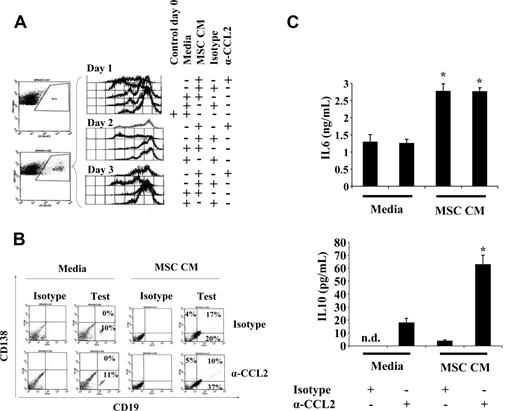
To explain the observed decreased number of ASCs after treatment with mpCCL2, we tested whether suppression of Ig production was the result of induced PC cell death. We found that mpCCL2 treatment of purified spleen-derived CD138+ cells did not lead to enhanced apoptosis compared with an untreated control group (data not shown). Indeed, in experimental work with unfractionated splenocytes, we observed an apparent mitogenic effect of mpCCL2 (data not shown). Therefore, we tested whether spleen-derived CFSE-labeled CD138+ PCs behaved similarly by measuring proliferative response to mpCCL2. Interestingly, MSC CM induced the proliferation of CD138+ cells as noticed by the loss of CFSE intensity over time, which could be reversed by CCL2-neutralizing antibody (Figure 3A). A significantly higher percentage of CD138/CD19 double-positive cells was also noticed at day 3 after MSC CM treatment in the absence of CCL2 neutralization as opposed to control groups, suggesting a specific induction of plasmablast proliferation by mpCCL2 (Figure 3B). Furthermore, interleukin (IL)–6 secretion was detected in MSC CM independent of CCL2 neutralization, whereas significantly higher levels of IL-10 were noticed after CCL2 blockade (Figure 3C). These findings are consistent with previous studies showing that CCL2 neutralization or CCR2 signaling blockade augmented IL-10 concentrations in serum during inflammation.18-22 Taken together, our data suggest that mpCCL2 present in MSC CM interacts with CCR2 leading to suppression of IL-10 production, PC dedifferentiation, and plasmablastic proliferation.
Biologic responses of PCs to mpCCL2. (A) MSC CM leads to CD19−CD138+ cell proliferation. After treatment of splenocytes with MSC CM, CD138+ gating demonstrates that the cells proliferate as of day 1, whereas the addition of CCL2 neutralizing antibody blocks this proliferative activity. The same profile was noticed for days 2 and 3. (B) MSC CM leads to plasmablast proliferation. No apparent CD138/CD19 double-positive cells could be found after a 3-day culture with splenocyte media (control group). However, a high percentage of plasmablast is detectable (17%) when cultured with MSC CM with a decrease to 10% after CCL2 neutralization. (C) CCL2 neutralization leads to IL-10 secretion. From the same experiments performed in panel A, IL-6 and IL-10 ELISAs were performed. IL-6 does not seem to be altered if CCL2 is neutralized, whereas IL-10 is up-regulated (n = 3/group; P < .005).
Biologic responses of PCs to mpCCL2. (A) MSC CM leads to CD19−CD138+ cell proliferation. After treatment of splenocytes with MSC CM, CD138+ gating demonstrates that the cells proliferate as of day 1, whereas the addition of CCL2 neutralizing antibody blocks this proliferative activity. The same profile was noticed for days 2 and 3. (B) MSC CM leads to plasmablast proliferation. No apparent CD138/CD19 double-positive cells could be found after a 3-day culture with splenocyte media (control group). However, a high percentage of plasmablast is detectable (17%) when cultured with MSC CM with a decrease to 10% after CCL2 neutralization. (C) CCL2 neutralization leads to IL-10 secretion. From the same experiments performed in panel A, IL-6 and IL-10 ELISAs were performed. IL-6 does not seem to be altered if CCL2 is neutralized, whereas IL-10 is up-regulated (n = 3/group; P < .005).
mpCCL2 inhibits AKT and STAT3 phosphorylation while inducing PAX5 in PCs
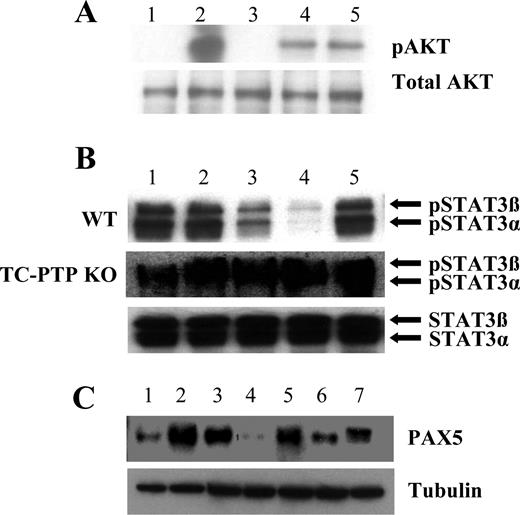
Our data support the hypothesis that mpCCL2 signals through the CCR2 receptor in a manner distinct from that of agonist, full-length CCL2. Examining signal transduction downstream of CCR2, we observed lower pAKT levels on sorted PCs (CD19−CD138+) after mpCCL2 treatment or during the addition of both full-length and mpCCL2 in 1:1 ratio (Figure 4A). A striking observation was the inhibition of STAT3 phosphorylation in CD19−CD138+ PCs after mpCCL2 or MSC CM treatments (Figure 4B). With the use of T-cell protein-tyrosine phosphatase (TC-PTP)−/− CD19−CD138+ PC, we show that this inhibition is dependent on the phosphatase TC-PTP, known to directly interact with phosphorylated STAT3,23 suggesting that mpCCL2 promotes TC-PTP–dependent STAT3 dephosphorylation once it interacts with CCR2. STAT3 activation in PC is crucial for antibody production24 because it leads to the expression of BLIMP-1, an important repressor of the transcription factor PAX5 to allow Ig synthesis.25-28 Concordant with the STAT3 data, PAX5 protein levels were increased in the presence of MSC CM or mpCCL2 but were repressed after CCL2 neutralization (Figure 4C).
Biochemical responses of PCs to mpCCL2. (A) Antagonist CCL2 acts as a dominant-negative molecule for AKT phosphorylation (pAKT). Lane 1 indicates unstimulated; lane 2, rCCL2; lane 3, rhMMP1; lane 4, mpCCL2; lane 5, rCCL2 plus mpCCL2 (1:1). rCCL2 stimulation of CD138+ PCs leads to pAKT, whereas mpCCL2 alone or in combination with full-length CCL2 leads to a reduced AKT activation demonstrating antagonistic activities occurring at the level of CCR2. (B) Antagonist CCL2 blocks STAT3 activation. Whole-cell lysate of CD138+ sorted cells was tested for pSTAT3 as follows: lane 1 indicates unstimulated control; lane 2, rCCL2; lane 3, cCCL2; lane 4, MSC CM plus isotypes; lane 5, MSC CM in the presence of CCL2-neutralizing antibody. pSTAT3 decreased after mpCCL2 stimulation but was completely absent on the addition of MSC CM. The pSTAT3 was induced after the addition of CCL2-neutralizing antibody. In addition, PCs derived from TC-PTP−/− splenocytes were refractory to STAT3 dephosphorylation by mpCCL2 as no changes were noticed after treatment. (C) mpCCL2 induces PAX5 expression in PC. Lane 1 indicates unstimulated; lane 2, MSC CM + iso; lane 3, MSC CM + α-CCL2; lane 4, rhMMP1; lane 5, rCCL2 + rhMMP1 + iso; lane 6, rCCL2 + rhMMP1 + α-CCL2; lane 7, A2B5 (positive control for PAX5). PAX5 was mainly detected in the lysate of sorted PCs after cCCL2 and MSC CM treatments, whereas CCL2 neutralization significantly decreases PAX5 levels. IgG blockade in PCs is not the result of apoptosis. To confirm that IgG inhibition in PCs is not the result of apoptosis induced by antagonist CCL2, PCs treated with media or mpCCL2 led to the same amount of cell death (25% vs 26%; data not shown).
Biochemical responses of PCs to mpCCL2. (A) Antagonist CCL2 acts as a dominant-negative molecule for AKT phosphorylation (pAKT). Lane 1 indicates unstimulated; lane 2, rCCL2; lane 3, rhMMP1; lane 4, mpCCL2; lane 5, rCCL2 plus mpCCL2 (1:1). rCCL2 stimulation of CD138+ PCs leads to pAKT, whereas mpCCL2 alone or in combination with full-length CCL2 leads to a reduced AKT activation demonstrating antagonistic activities occurring at the level of CCR2. (B) Antagonist CCL2 blocks STAT3 activation. Whole-cell lysate of CD138+ sorted cells was tested for pSTAT3 as follows: lane 1 indicates unstimulated control; lane 2, rCCL2; lane 3, cCCL2; lane 4, MSC CM plus isotypes; lane 5, MSC CM in the presence of CCL2-neutralizing antibody. pSTAT3 decreased after mpCCL2 stimulation but was completely absent on the addition of MSC CM. The pSTAT3 was induced after the addition of CCL2-neutralizing antibody. In addition, PCs derived from TC-PTP−/− splenocytes were refractory to STAT3 dephosphorylation by mpCCL2 as no changes were noticed after treatment. (C) mpCCL2 induces PAX5 expression in PC. Lane 1 indicates unstimulated; lane 2, MSC CM + iso; lane 3, MSC CM + α-CCL2; lane 4, rhMMP1; lane 5, rCCL2 + rhMMP1 + iso; lane 6, rCCL2 + rhMMP1 + α-CCL2; lane 7, A2B5 (positive control for PAX5). PAX5 was mainly detected in the lysate of sorted PCs after cCCL2 and MSC CM treatments, whereas CCL2 neutralization significantly decreases PAX5 levels. IgG blockade in PCs is not the result of apoptosis. To confirm that IgG inhibition in PCs is not the result of apoptosis induced by antagonist CCL2, PCs treated with media or mpCCL2 led to the same amount of cell death (25% vs 26%; data not shown).
MSC administration lowers OVA-specific IgG titer in vaccinated mice
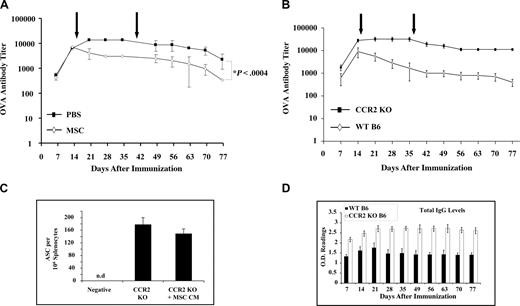
In light of the inhibitory effects of MSC on activated PC production of Ig, we examined the effect of administering exogenous syngeneic MSCs in xenoantigen–immunized mice. We found that rOVA-immunized mice responded to MSC administration with significantly decreased anti-OVA IgG titers (Figure 5A). Because mpCCL2 interacts with CCR2, we presumed that CCR2 receptor expression by target PCs is required to inhibit IgG secretion. Therefore, rOVA-immunized CCR2−/− mice were given MSCs. Not only did MSC injection fail to modulate IgG titers in vivo, but these mice developed a stronger anti-OVA humoral response than the WT control group (Figure 5B). Furthermore, spleen-derived PCs from CCR2−/− mice were refractory to MSC CM effect in vitro (Figure 5C). However, the fact that overall IgG levels in both WT and CCR2−/− mice were not altered after MSC delivery suggests that the cellular therapy is specific for CCR2-expressing activated PC only and not all Ig-producing PCs (Figure 5D).
MSC injection in vivo decreases IgG titers. (A) MSC injection in OVA-immunized mice can lead to faster clearance of IgG titers. After immunization of B6 mice with rOVA, MSCs were injected intraperitoneally at 4-week intervals and bled weekly for anti-OVA IgG titer by ELISA. MSCs led to a faster decrease in IgG levels as opposed to the control group. (B) ELISPOT assay using CCR2−/− cells. CCR2−/− mice are not affected by MSCs. CCR2−/− mice were immunized with rOVA along with WT B6 mice and injected with MSC as explained in panel A. High IgG titers were developed in CCR2−/− with no significant decrease after MSC injection. (C) Total IgG titers. In vitro ELISPOT performed with CCR2−/− and WT B6 splenocytes demonstrates that CCR2−/− cells are refractory to antagonist CCL2 (n = 3/group). (D) Total IgG titers. Total IgG levels screened by ELISA shown that the overall circulating IgGs were not affected by MSC injection.
MSC injection in vivo decreases IgG titers. (A) MSC injection in OVA-immunized mice can lead to faster clearance of IgG titers. After immunization of B6 mice with rOVA, MSCs were injected intraperitoneally at 4-week intervals and bled weekly for anti-OVA IgG titer by ELISA. MSCs led to a faster decrease in IgG levels as opposed to the control group. (B) ELISPOT assay using CCR2−/− cells. CCR2−/− mice are not affected by MSCs. CCR2−/− mice were immunized with rOVA along with WT B6 mice and injected with MSC as explained in panel A. High IgG titers were developed in CCR2−/− with no significant decrease after MSC injection. (C) Total IgG titers. In vitro ELISPOT performed with CCR2−/− and WT B6 splenocytes demonstrates that CCR2−/− cells are refractory to antagonist CCL2 (n = 3/group). (D) Total IgG titers. Total IgG levels screened by ELISA shown that the overall circulating IgGs were not affected by MSC injection.
MSC administration to hemophilic mice lowers hFVIII-specific IgG titer
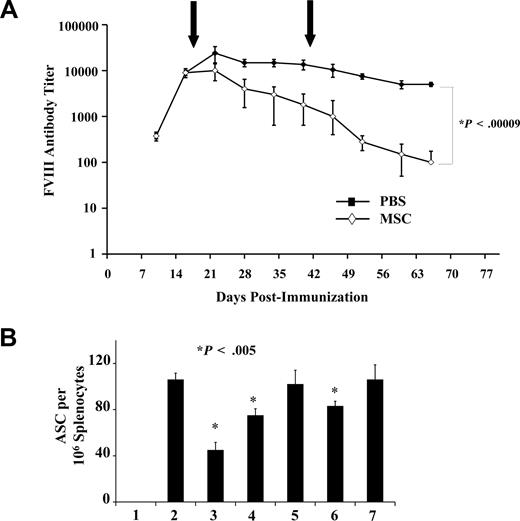
Acquired alloantibodies directed against therapeutic proteins, such as recombinant hFVIII used in the treatment of hemophilia A, can be a serious complication to therapy.29 To test the hypothesis that MSCs can help decrease the level of neutralizing alloantibodies, we induced anti-hFVIII antibodies in B6 hemophilic mice and subsequently administered syngeneic MSCs, and observed a near 2-log decrease in anti-hFVIII IgG titer (Figure 6A). ELISPOT assays performed on spleen-derived ASCs from hemophilic mice showed a decreased number of anti-hFVIII Ig-secreting PC after treatment with WT MSC CM and, to a lesser extent, with CCL2−/− MSCs. The weak inhibition seen by CCL2−/− MSC CM was the result of CCL7 secretion because its neutralization blocked the observed anti-hFVIII Ig-secreting PC inhibition (Figure 6B).
MSC effect on pathologic FVIII response. (A) Faster clearance of hFVIII IgG on MSC injection in hemophilic mice. hFVIII-immunized hemophilic B6 mice were injected with MSCs intraperitoneally after the same scheme as in panel A. The IgG profile shows high IgG clearance compared with the nontreated group. (B) ELISPOT assay using FVIII-derived splenocytes. In vitro ELISPOT performed with immunized hemophilic B6 mice splenocytes demonstrates that CCL2 neutralization rescues approximately 75% of the IgG secretion ability by PC, whereas simultaneous inhibition of CCL2/7 completely restored Ig secretion. Lane 1 indicates negative; lane 2, positive; lane 3, MSC CM + isotype; lane 4, MSC CM + CCL2 neutralizing antibody; lane 5, MSC CM + neutralizing CCL2/7 antibodies; lane 6, CCL2−/− MSC CM + isotype; lane 7, CCL2−/− MSC CM + CCL7 neutralizing antibody.
MSC effect on pathologic FVIII response. (A) Faster clearance of hFVIII IgG on MSC injection in hemophilic mice. hFVIII-immunized hemophilic B6 mice were injected with MSCs intraperitoneally after the same scheme as in panel A. The IgG profile shows high IgG clearance compared with the nontreated group. (B) ELISPOT assay using FVIII-derived splenocytes. In vitro ELISPOT performed with immunized hemophilic B6 mice splenocytes demonstrates that CCL2 neutralization rescues approximately 75% of the IgG secretion ability by PC, whereas simultaneous inhibition of CCL2/7 completely restored Ig secretion. Lane 1 indicates negative; lane 2, positive; lane 3, MSC CM + isotype; lane 4, MSC CM + CCL2 neutralizing antibody; lane 5, MSC CM + neutralizing CCL2/7 antibodies; lane 6, CCL2−/− MSC CM + isotype; lane 7, CCL2−/− MSC CM + CCL7 neutralizing antibody.
Discussion
We have demonstrated that the MSC secretome has the capacity to decrease the number of spleen-derived, antigen-specific ASCs from rOVA-immunized mice. However, this inhibitory property was lost on MSC fixation before splenocyte treatment, implying that metabolically active MSCs are required for the generation of inhibitory soluble factors. Although NO was identified as an inhibitor of activated T cells after a direct MSC/T-cell contact,10,17 we found that it played no role in modulating ASCs in vitro. However, we identified at least 2 CC chemokines (CCL2 and CCL7) produced by MSCs that represent the sum total of the suppressor activity contained in MSC secretome. More specifically, the MMP-processed variant of CCL2 and possibly CCL7 is the key B-cell regulatory effector molecule. This discovery gives some insight on how MSCs, as part of the marrow microenvironment, may play a role in humoral immune response homeostasis.
B-cell maturation is a process that occurs both in spleen and bone marrow to form fully mature naive B cells.30 On antigen stimulation, germinal centers appear in the spleen, leading to the generation of plasmablast and ultimately PCs, which do not divide but secrete antibodies.31 These developmental stages are controlled by a variety of transcription factors, such as E2A and EBF, which coordinately induce the expression of B-cell lymphoid genes at least at the early progenitor stage.30 However, this early progenitor commitment to the B-lymphoid lineage is dependent on PAX5, which was identified as the key regulator of B-cell identity.32 More specifically, a Pax5 mutation is capable of arresting B-cell development, whereas its overexpression in Pax5−/− pro-B cells leads to the formation of mature B cells.33 In addition, PAX5 must be repressed to allow PC differentiation because it directly represses the X-box–binding protein 1 (XBP1), IgH, IgL, and the IgJ chain34-37 in addition to more than 100 repressed genes.38 One of the most important reactivated genes on PAX5 repression is CCR2 (the sole receptor for CCL2), which was proven to be important for PC differentiation and function.38 Thus, a dynamic machinery of regulation is involved for the generation of fully functional antibody-secreting PCs. Successful immune reactions lead to the accumulation of PCs in inflamed tissues, also known as survival niches until the antigen of interest is fully neutralized. At that stage, the elimination of survival signals in these niches leads to PC death and eventually a decrease of circulating antibody titers.39 In parallel, a proportion of plasmablasts migrates to the bone marrow and eventually becomes immobile long-lived PCs for further secondary responses on antigen restimulation.40 MSCs in the bone marrow are known to be involved at the early stages of B-cell development and PC homeostasis. We here demonstrate that MSC production of MMP-processed CCL2 may represent a key molecular event in the paracrine modulation of PC production of Igs. Indeed, during PC terminal differentiation, PAX5 is repressed, therefore allowing the reactivation and expression of ccr2,38 which could eventually represent a receptor of choice for modulation by MSC-derived CCL2 and derivatives.
The biologic activity of CCL2 can be modulated via various mechanisms.41 For example, the glycosylation pattern leading to chemokines with a molecular weight of 12 or 13.5 kDa can decrease by 2- to 3-fold the chemotactic activity of CCL2 on primary monocytes while enhancing their half-life.41 In addition, it was also found that N-terminal processing of CCL2 by MMPs leading to variants 5-76 or 6-76 was practically devoid of agonist bioactivity when tested for their ability to trigger Ca2+ influx in CCR2-expressing cells.41 Furthermore, C-terminally processed CCL2 (1-69), on the other hand, fully retained its chemotactic and Ca2+-inducing capacity compared with variant 5-76 or 6-76, suggesting very complex regulation mechanisms on CCL2 activity.41 Compared for their antagonistic capacities, only variant 5-76 could compete with full-length CCL2 for binding to the receptor as opposed to the 6-76 truncated form.41 Because MSC CM contains an antagonist variant capable of inhibiting pAKT or STAT3, we predict that the variant present in MSC CM is an N-terminal truncated form, acting as dominant negative signaling molecule on CCR2.
Several signals alone or in combination, such as IL-2, IL-4, IL-5, IL-6, and IL-10, are known to support the survival of PCs.42 Among many, IL-6 was thought to be the most important survival factor because of its capacity to support PC survival ex vivo.42 However, it was then discovered that IL-6-deficient mice had no defects in the maintenance of humoral memory responses.42,43 Our finding that MMP-processed CCL2 triggers the proliferation of plasmablasts in vitro in an IL-6–independent manner correlates perfectly with this model. Because plasmablasts are substantially less efficient than fully differentiated PCs at producing Igs, we speculate that this explains the robust reduction of alloantigen IgG titers we observed in vivo. We also noted that PCs treated with MSC CM or cleaved mpCCL2 in vitro do not undergo increased apoptosis relative to controls (data not shown), which supports the notion that Ig suppression is not achieved through PC killing but rather by their conversion to a low Ig secretor phenotype. To address mechanistically this observation, we performed a series of in vitro and in vivo experiments. First, we found that CCR2−/− mice are utterly unresponsive to the suppressive effects of MSCs on antigen-specific Ig production, suggesting that all downstream effects of MMP-processed CCL2 on Ig-producing cells occur through receptor interaction with CCR2 only. Focusing on the biochemical responses of PCs after MSC CM or in vitro MMP1-cleaved CCL2 treatment, we obtained a robust dephosphorylation of STAT3 in a TC-PTP–dependent manner. It was previously reported that conditional STAT3−/− in PCs blocks antibody synthesis of the IgG isotype because it directly affects the expression of BLIMP-1, allowing an additional derepression mechanism on PAX5 expression.24 Furthermore, we observed that the presence of antagonist CCL2 in MSC CM suppressed IL-10 production by splenocytes in vitro. Because IL-10 normally induces BLIMP-1 and plays an important role in the humoral immune response,44 its blocked production can also account in part for the observed IgG inhibition. As a result, the synergizing effects of IL-10 production blockade and STAT3 dephosphorylation lead to de novo expression of PAX5 in PCs, dedifferentiation to plasmablasts, and suppressed Ig production.
Because PCs express CCR2 and can be involved in maladapted immune responses exemplified by autoimmune diseases,29,44 we hypothesized that the inhibition of PCs via CCL2 antagonism could allow a robust blockade of Ig secretion and thus alleviate clinically relevant humoral pathologies. To test this idea, we alloimmunized hemophilic mice to human recombinant factor VIII. These mice develop neutralizing anti-FVIII antibodies akin to what is seen in more than 30% of hemophilia A sufferers treated with recombinant FVIII replacement therapy.29 We tested whether autologous MSCs could reduce the anti-FVIII titers after alloimmunization and observed a significant and stable decrease in anti-FVIII IgG titers, yet total IgG levels remained normal and unaffected. This inhibition was lost if CCR2−/− mice were used in addition to the fact that a higher IgG titer was obtained in CCR2−/− compared with WT mice.
How can we conciliate suppression of alloantibodies without a substantial effect on total IgG levels? It is well known that helper CD4 T cells are implicated in providing T-cell help for the initiation of a humoral response.45 Interestingly, CCR2 has been reported to be absent on naive lymphocytes and up-regulated on activated T cells (both CD4 and CD8) as reported by Rabin et al.45 Thus, we propose that only activated CD4 T cells implicated in the ongoing Th2 help will be targeted by mpCCL2 and inhibited. In addition, PCs were reported by Delogu et al to up-regulate CCR2 as well.37 As such, the MSC-derived antagonist CCL2 would target both Th2 and CD4 T cells, providing the T-cell help as well as CCR2+ PCs secreting anti-FVIII antibodies without affecting quiescent memory B or T cells. Therefore, it is possible that there is a measure of selectivity in Ig suppression, based on CCR2 expression by “activated” in contrast to steady-state or quiescent lymphoid targets.
In conclusion, the development of antigen-specific Ig-producing PC is fundamental to humoral response. Our observations demonstrate that MSCs secrete and process CCL2 generating a molecule with profound inhibitory effects on PCs ability to secrete Ig because of TC-PTP–dependent STAT3 inhibition and PAX5 induction. The induction of PAX5 is probably transcriptional in origin.46,47 In addition, the specific proliferation of plasmablast is favored to the detriment of PCs, whereas IL-10 levels are low in the presence of mpCCL2. As a result, plasmablasts may compete for the bone marrow or survival niche affecting overall Ig production as demonstrated by the study of Odenhal et al.38 Thus, through its MMP-dependent extracellular proteolytic processing of CC chemokine CCL2 and possibly, to a lesser extent, CCL7, marrow-resident MSCs may play an important physiologic role in Ig response and Th2 homeostasis. It must be noted that culture-expanded polyclonal MSCs are heterogeneous and there are intergroup variances in their analysis, most probably the result of various reasons, such as number of passages, cell confluence, handling, and culture media. In regards to CCL2 expression, we and other groups have identified CCL2 as a chemokine produced by MSCs, and this from an array of mouse strains (C57BL/6 and BALB/c) and from human MSCs.48 This reinforces the notion that CCL2 is of a common ground between MSC populations derived from multiple mouse strains and from humans as well and does not represent an idiosyncratic artifact of the MSCs used by our group. As for MSC efficacy in vivo, the recent success in the use of MSCs to treat steroid-resistant GVHD, as reported by Le Blanc et al,49 allows one to predict that the infusion of human MSCs in patients with a maladaptive humoral ailment is of great potential as well. The exact mechanism of action by which MSCs suppress GVHD remains unknown. Interestingly, it has been demonstrated by Terwey et al that donor CCR2+ CD8 effector lymphocytes are mandatory for a GVHD response.50 It is conceivable that the MSC CCR2-dependent mechanism of action we have here uncovered may be operative in suppression of alloreactive T cells as well. We can therefore speculate that autoimmune and alloimmune humoral and cell-mediated ailments driven by the CCL2/CCR2 axis, such as acute and chronic GVHD diseases driven by pathologic autoantibodies and the like, would be responsive to MSC-based cell therapies. These observations and our data regarding suppression of antigen-specific alloimmune response to ovalbumin and FVIII open up the possibility of exploiting MSCs as part of a cell therapy approach to interfere with the development of pathogenic alloantibodies or autoantibodies as well.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr G. E. Rivard for kindly providing rhFVIII for our studies.
This work was supported by the Canadian Institute of Health Research (grant MOP-15017). M.R. is a recipient of a Fonds de Recherches en Santé du Québec Scholarship, and J.G. is a Fonds de Recherches en Santé du Québec Chercheur-Boursier Sénior.
Authorship
Contribution: M.R. designed the research plans, performed immunization, cell culture, flow cytometric analysis, MLRs, ELISPOTs, and ELISAs, and wrote the manuscript; J.H. performed ELISPOTS and cell culture; S.F. performed Western blots for MMPs; M.Y.L. performed cell culture; S.Y. performed Tricine gels for the detection of CCL2; E.B. and K.F. handled the animals; M.-N.B. performed RT-PCR; K.D. handled the TC-PTP−/− mice; M.T. supplied the TC-PTP−/− mice; B.A. designed experiments for MMPs; and J.G. designed research plans and was the senior author.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacques Galipeau, Division of Hematology/Oncology, Jewish General Hospital, McGill University, 3755 Cote Ste-Catherine Road, Montreal, QC, Canada H3T 1E2; e-mail: jacques.galipeau@mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal