Abstract
In vitro studies have implicated chemokine receptors in consumption and clearance of specific ligands. We studied the role that various signaling chemokine receptors play during ligand homeostasis in vivo. We examined the levels of ligands in serum and CNS tissue in mice lacking chemokine receptors. Compared with receptor-sufficient controls, Cx3cr1−/− mice exhibited augmented levels of CX3CL1 both in serum and brain, and circulating levels of CXCL1 and CXCL2 were increased in Cxcr2−/− mice. CCR2-deficient mice showed significantly increased amounts of circulating CCL2 compared with wild-type mice. Cxcr3−/− mice revealed increased levels of circulating and brain CXCL10 after experimental autoimmune encephalomyelitis (EAE) induction. CCR2-deficient peripheral blood and resident peritoneal cells exhibited reduced binding capacity and biologic responses to the CCR1 ligand CCL3, suggesting that elevated levels of CCR2 ligands had down-regulated CCR1. The results indicate that signaling chemokine receptors clear chemokines from circulation and tissues. These homeostatic functions of signaling chemokine receptors need to be integrated into safety and efficacy calculations when considering therapeutic receptor blockade.
Introduction
Actions of chemokines through chemokine receptor signaling leads to an array of diverse functions in different tissue compartments.1,2 Such functions go beyond the original assigned roles of chemokines in leukocyte chemoattraction to inflamed tissues, and involve physiological trafficking to localize surveillant populations in noninflamed tissues, cellular activation, proliferation, adhesion, phagocytosis, apoptosis, and angiogenesis.3-6 Chemokine/chemokine receptor interactions exhibit defined roles during inflammation, atherosclerosis, autoimmunity, viral pathogenesis, cancer, and neurodegeneration.7-11 Even though the system exhibits apparent redundancy, modulation of chemokine function via chemokine receptor blockade is a challenging area of considerable interest for therapeutic purposes.
Among the chemokine receptors, CCR2 and its ligand CCL2 (MCP-1) have been extensively studied, and their role in regulating monocyte and T-cell infiltrations is well established. In 2002, Tylaska et al showed that CCR2-knockout mice manifested extremely high levels of CCL2 at sites of alloinduced inflammation, and in vitro studies confirmed that clearance of ligand was mediated by CCR2.12 Our group reported that CCL2 is consumed by CCR2+ migrating cells in a human blood-brain barrier model using peripheral blood mononuclear cells from healthy donors.13 These results suggest an important biologic role of chemokine receptors as scavenger molecules involved in clearance of specific ligands.
Chemokine receptor–deficient mouse strains have been instrumental in understanding chemokine biology in health and disease states.14 In the present study, we evaluated levels of chemokines in 4 receptor-deficient mice, including those lacking CC, CXC, and CX3C receptors. Both circulating and brain tissue levels were studied. Brain was selected for study as a distinct tissue compartment in which chemokines may be produced under physiological and pathological conditions. We found that the levels of circulating and—in some instances—tissue chemokines are dramatically increased in healthy chemokine receptor–deficient mice. Reconstitution with wild-type bone marrow cells restored chemokine homeostasis. Importantly, for chemokines that signal to more than one receptor, absence of one receptor, leading to high levels of circulating chemokine reduced the availability of alternate receptors probably due to ligand-mediated desensitization. Therefore, in addition to other functions, chemokine receptors play a homeostatic role by clearing chemokines from the circulation and tissues. Our findings may be relevant for interpreting studies involving chemokine receptor blockade. Blocking chemokine receptors in humans might produce analogous effects to chemokine receptor gene targeting in mice, and the biologic significance of high levels of circulating chemokines therefore needs to be addressed.
Methods
Mice
All mouse lines, including wild-type C57BL/6, Cx3cr1, Cxcr2, Cxcr3, and Ccr2 chemokine receptor–deficient strains, were obtained from our breeding colony at the Biological Resources Unit, Cleveland Clinic, Lerner Research Institute. Cx3cr1−/−Ccr2−/− double-knockout mice were generating by crossing Cx3cr1−/− with Ccr2−/− mice and extensively breeding the resulting Cx3cr1+/−Ccr2+/− progeny. Mouse lines were backcrossed to C57BL/6 for at least 11 generations, except the Cxcr2 line that is backcrossed to the SJL/J background. Animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic, and following the National Institutes of Health guidelines for animal care.
Mice were genotyped by polymerase chain reaction (PCR) using tail DNA, and chemokine receptor–specific primers (Invitrogen, Carlsbad, CA) (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). PCR reactions were prepared in a volume of 10 μL using AmpliTaq-Gold system (Applied Biosystems, Foster City, CA) and resolved in 1% agarose gels.
EAE induction
Wild-type and Cxcr3−/− littermate female mice 8 to 10 weeks old were immunized subcutaneously in the flanks with 100 μg MOG (33-55) peptide emulsified in complete Freund adjuvant containing 4 mg/mL Mycobacterium tuberculosis H37 RA (DIFCO, Detroit, MI).15 Pertussis toxin (200 ng) was injected intraperitoneally on the day of immunization and repeated 48 hours later. Mice were weighed daily and monitored for signs of disease according to the following parameters16 : 0 indicates no disease; 1, decreased tail tone and/or poor righting ability; 2, tail atony; 3, partial limb paralysis; 4, complete limb paralysis; 5, ascending paralysis; and 6, death. Mice were killed when a score of 4 was reached.
Brain chemokine measurements by ELISA
For the detection of soluble chemokines in CNS tissues, mice were anesthetized with a lethal dose (2 mg/mouse) of pentobarbital and intracardially perfused with ice cold Hanks balanced salt solution (Invitrogen). Brains were dissected and disrupted manually using dounce homogenizers. Cell suspensions were made in buffer (2 mL/brain) containing 150 mM NaCl, 0.01 M Tris, 1.0 mM EDTA, 1.0 μg/mL aprotinin, and 100 μg/mL PMSF. Tissue lysates were centrifuged at 500g for 10 minutes at 4°C and supernatants aliquoted and stored at −80°C. Total protein concentrations were obtained using the Bio-Rad protein reagent assay (Bio-Rad, Hercules, CA), and the quantities of soluble chemokines CX3CL1, CCL2, CXCL1, CXCL2, and CXCL10 were measured by enzyme-linked immunosorbent assay (ELISA) using the murine Duo Set development systems (R&D Systems, Minneapolis, MN). Each sample was assayed in 2 different dilutions and run in duplicate. Results are reported as picogram amounts of chemokine per milligram of protein.
Detection of CX3CL1 mRNA by quantitative real time RT-PCR
Wild-type and Cx3cr1−/− mice were perfused with HBSS, the cerebral cortex was dissected, and total RNA was isolated using TRizol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA was cleaned up using the RNeasy mini kit (Qiagen, Valencia, CA) and was measured by spectrophotometry, and integrity was assayed by visualization in 1% agarose gels. Quantitative reverse transcription (RT, Superscript II reverse transcriptase kit; Invitrogen)–coupled PCR assays were performed using LightCycler (Roche, Indianapolis, IN) as described previously. Primers CX3CL1-F: ATT GTC CTG GAG ACG ACA CAG C and CX3CL1-R: TTG CCA CCA TTT TTA GTG AGG G were used for the detection of CX3CL1 expression. GAPDH expression was used as an internal control for each sample. A standard curve was generated using the known amounts of the purified PCR product using the primers CX3CL1-F and CX3CL1-R, and results were expressed as femtogram of CX3CL1 product.
Serum collection
Mice were bled via the cheek pouch on the submandibular vein using 4-mm goldenrod lancet. Blood (200-300 μL) was allowed to clot at 4°C for 4 hours and centrifuged at 2000g for 20 minutes at 4°C. Serum was removed and stored at −20°C in the presence of complete protease inhibitor cocktail (Roche).
Generation of bone marrow chimerae
Recipient mice (5-6 weeks old) were irradiated with a dose of 9 Gy, according to Cleveland Clinic guidelines, and allowed to recover for 3 to 5 hours before bone marrow reconstitution. For the isolation of bone marrow cells, donor mice were killed by CO2 asphyxiation, the entire legs were dissected, and bone marrow cells were flushed from femur and tibia with Iscove media (Invitrogen) supplemented with 10% fetal bovine serum (Atlas, Biologicals, Fort Collins, CO) and 50 μg/mL gentamycin (Invitrogen). Bone marrow cells were spun at 1000g for 7 minutes at 4°C and cell pellets resuspended in Iscove media without FBS at 15 × 107 cells/mL. Recipient mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (5 mg/kg), and 15 to 20 × 106 cells were injected via the retro-orbital sinus in a volume of 150 to 200 μL. Mice were placed on a clean cage and monitored until they were fully awake. Four weeks after bone marrow reconstitution, mice were bled via the submandibular vein as described above, and heparinized blood was processed for DNA isolation using DNeasy spin columns according to the manufacturer's instructions (Qiagen). Chimeric mice were allowed to reconstitute for 6 weeks after bone marrow transfer prior to analyses of tissues.
In vitro chemokine clearance assay
Tissues from Cx3cr1+/+, Cx3cl1−/−, and Cx3cr1−/− mice were perfused with cold HBSS and 300-μm vibrotome sections were obtained. Cortical tissues (3 per well) were incubated for 1 hour at 4°C or 37°C/5% CO2, in DMEM media containing 10% horse serum and 50 μg/mL gentamycin, with or without mouse recombinant CX3CL1 (R&D Systems) at 5000 pg/mL. Culture media were collected and stored at −20°C, and the amount of CX3CL1 in media was assayed as described in “Brain chemokine measurements by ELISA.”
Quantitative determination of cells bearing surface CCL3 receptors
Whole blood was collected by cardiac puncture in heparinized tubes and red blood cells were removed by ficoll sedimentation. The interphase containing peripheral blood mononuclear cells (PBMCs) was washed twice in 10 mL HBSS. Resident peritoneal cells were collected by peritoneal lavage in 10 mL sterile PBS and centrifuged at 700g for 7 minutes at 4°C. PBMCs and peritoneal macrophages were resuspended at 4 × 106 cells/mL and treated with anti–mouse CD16/CD32 (Fcγ III/II receptor). Cells were incubated with biotinylated CCL3 reagent, or with a preincubated mix of biotin-CCL3 and CCL3 blocking antibody as a specificity control according to the manufacturer's instructions (Fluorokine, mouse CCL3 biotin conjugate; R&D Systems). Samples were then incubated with avidin-FITC reagent, anti–mouse CD11b-PE (clone M1/70; BD Pharmingen, San Diego, CA), and anti–mouse CD3-PercP (clone 145–2C11; BD Pharmingen).
In vitro chemotaxis assay
Resident peritoneal cells (50 000 cells/well) were placed in the top compartment above a polycarbonate membrane containing 8.0-μm pores, and 600 μL medium or chemoattractant (CCL3, CXCL12) was placed in the lower compartment, in 24-well plates as described.17 Cells were incubated 2 hours at 37°C, and the membrane was washed (top side only)17 and mounted in media containing 450 nM DAPI. Experiments were performed in triplicate for each chemoattractant concentration. For each well, cells located on the bottom side of the membrane (cells that have undergone chemotaxis through the membrane) were counted in 10 random fields chosen from the center and all 4 peripheral quadrants (magnification, 200×). Final results represent the mean of 2 independent experiments. Data are expressed as percentage migration, which represents the fold increase in the number of cells migrating in response to chemoattractants (EG indicates experimental group) over the cell response to the control medium (CG indicates control group); % migration = (EG/CG)(100).
Statistical analyses
Results were compared using an unpaired t test with GraphPad Prism software (San Diego, CA).
Results
CX3CR1-deficient mice exhibit increased levels of CX3CL1 in the CNS and in peripheral blood
To determine whether the absence of CX3CR1 had an effect on the amount of ligand present in the brain, we measured soluble CX3CL1 levels by ELISA (Table 1) in aqueous extracts obtained from brain homogenates from healthy adult mice (8-12 weeks old). Mice lacking CX3CR1 showed a 30-fold excess of soluble CX3CL1 in the brain compared with wild-type littermates (P ≤ .001). Cx3cr1−/− mice exhibited remarkably high levels of serum CX3CL1 (300-fold increase over levels present in wild-type mice, P = .003).
Quantitation of CX3CL1 in brain and serum of healthy mice
| Tissue/genotype . | Cx3cr1+/+ . | Cx3cr1−/− . | Fold change . |
|---|---|---|---|
| Brain | |||
| Protein, pg* | 1500 ± 800 | 48000 ± 300† | > 30 |
| mRNA, fg | 44 ± 10 | 43 ± 10 | 1 |
| Blood, protein, pg* | 780 ± 200 | 191000 ± 80000‡ | > 300 |
| Tissue/genotype . | Cx3cr1+/+ . | Cx3cr1−/− . | Fold change . |
|---|---|---|---|
| Brain | |||
| Protein, pg* | 1500 ± 800 | 48000 ± 300† | > 30 |
| mRNA, fg | 44 ± 10 | 43 ± 10 | 1 |
| Blood, protein, pg* | 780 ± 200 | 191000 ± 80000‡ | > 300 |
Soluble CX3CL1 was measured by ELISA in brain aqueous homogenates (n = 4 mice per group) and serum (n = 9 mice per group).
Data represent the mean plus or minus standard deviation (SD) in pg/mg protein. Brain and circulating levels were significantly higher in Cx3cr1−/− mice compared with wild-type mice.
P < .001,
P = .003. CX3CL1 message was quantified by real time RT-PCR, showing no statistical significance in brain mRNA expression; n = 4 mice per group.
Increased levels of CNS CX3CL1 are not caused by increased transcription
CX3CL1 is primarily expressed in the CNS by neurons. Increased CX3CL1 in the CNS of Cx3cr1−/− mice might be mediated by release of feedback inhibition, which would be reflected in increased gene transcription. CX3CL1 mRNA levels were comparable in CNS tissues of wild-type and Cx3cr1−/− mice (Table 1; P > .1). This result indicated that increased CX3CL1 was not caused by accumulation of mRNA.
Increased levels of CXCL1 and CXCL2 in CXCR2-deficient mice
CXCL1 is produced primarily in the adult CNS by astrocytes and CXCR2 is its unique receptor. CXCR2-deficient mice exhibited increased brain and blood CXCL1 levels, compared with Cxcr2+/+ littermates (Table 2; P = .008 for comparison of brain levels of CXCL1, and P < .001 for comparison of serum levels). We evaluated the levels of an additional CXCR2 ligand, and found that circulating levels of CXCL2 were undetectable in wild-type mice, contrasting with Cxcr2−/− mice, which had serum CXCL2 amounts greater than 200 pg/mg protein (Table 2).
Quantitation of CXCL1 and CXCL2 in brain and serum of healthy mice
| Ligand*/genotype . | Cxcr2+/+ . | Cxcr2−/− . | Fold change . |
|---|---|---|---|
| CXCL1 | |||
| Brain | 86 ± 19 | 362 ± 200† | 4 |
| Blood | 325 ± 39 | 5691 ± 2000‡ | 17 |
| CXCL2, blood | ND | 464 ± 136 | > 100 |
| Ligand*/genotype . | Cxcr2+/+ . | Cxcr2−/− . | Fold change . |
|---|---|---|---|
| CXCL1 | |||
| Brain | 86 ± 19 | 362 ± 200† | 4 |
| Blood | 325 ± 39 | 5691 ± 2000‡ | 17 |
| CXCL2, blood | ND | 464 ± 136 | > 100 |
Levels of the CXC chemokine, CXCL1 were measured by ELISA in brain supernatants (n = 6 mice per group) and serum (n = 9 mice per group). CXCL2 was measured in serum (n = 4 mice per group).
Data represent the mean ± SD of CXCL1 amount in pg/mg protein. Brain and circulating levels were significantly higher in Cxcr2−/− mice compared with wild-type mice.
P = .008.
P < .001.
CXCL10 detection in CXCR3-deficient mice after EAE induction
CXCL10, a CXCR3 ligand, was not detected in brain or serum of healthy mice, but is present at high levels in the CNS of mice with the inflammatory model disease experimental autoimmune encephalomyelitis (EAE). To determine whether CXCL10 homeostasis was altered in CXCR3-deficient mice, we induced EAE in wild-type and Cxcr3−/− mice. Brain tissue and serum samples were harvested on the day of peak disease. CXCL10 was detected in the serum of Cxcr3−/− mice, but not in wild-type mice (Table 3). We also detected a significant increase in brain CXCL10 levels in Cxcr3−/− mice with EAE compared with wild-type mice with EAE.
Measurement of CXCL10 in the brain and serum of mice at peak EAE
| CXCL10*/genotype . | Cxcr3+/+ . | Cxcr3−/− . | Fold change . |
|---|---|---|---|
| Brain | 1148 ± 131 | 1878 ± 190† | 1.6 |
| Blood | ND | 1157 ± 581 | >100 |
| CXCL10*/genotype . | Cxcr3+/+ . | Cxcr3−/− . | Fold change . |
|---|---|---|---|
| Brain | 1148 ± 131 | 1878 ± 190† | 1.6 |
| Blood | ND | 1157 ± 581 | >100 |
Levels of the CXCL10 were measured by ELISA in brain supernatants and serum (n = 6, Cxcr3+/+ mice and n = 4, Cxcr3−/− mice).
Data represent the mean plus or minus SD in pg/mg protein.
P = .01.
CCR2-deficient mice exhibit increased levels of circulating CCL2
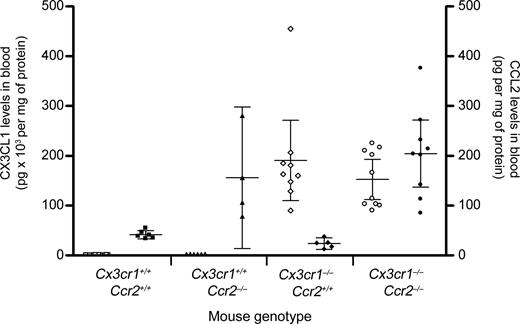
Mice lacking CCR2 exhibited a higher amount of circulating CCL2 (156 ± 89 ng/mL in Ccr2−/− mice compared with 41 ± 8 in Ccr2+/+, P = .01). Because mice lacking CCR2 exhibit altered circulating cell populations, we compared amounts of CX3CL1 and CCL2 in the serum of mice lacking either or both CX3CR1 and CCR2, thereby minimizing the effects of cell population shifts (Figure 1). Mean basal levels of circulating CX3CL1 in wild-type mice were less than 1 ng/mL (Table 1; 0.7 ± 0.2 ng/mL). In agreement with our previous observations, Cx3cr1−/− and Cx3cr1−/−Ccr2−/− double-knockout mice manifested levels of circulating CX3CL1 that were significantly higher compared with wild-type mice (190.9 ± 80.5 ng/mL CX3CL1 in Cx3cr1−/−; P < .001, and 153 ± 99 ng/mL in Cx3cr1−/−Ccr2−/−; P = .002). Importantly, the amount of circulating CX3CL1 in Cx3cr1−/− and Cx3cr1−/−Ccr2−/− mice was comparable (P = .2). These results supported the hypothesis that chemokine receptors play a role in homeostatic mechanisms that regulate ligand levels in blood and tissues.
Comparative analysis of circulating CX3CL1 and CCL2 levels. Serum samples from Cx3cr1−/−, Ccr2−/−, and Cx3cr1−/−Ccr2−/− double-knockout mice were assayed by ELISA for the presence of CX3CL1 and CCL2, and results are shown in the left y-axis (open symbols) and right y-axis (filled symbols), respectively. Bars show mean value with 95% confidence interval (CI) of chemokine in pg/mg of protein. Each point represents an individual mouse.
Comparative analysis of circulating CX3CL1 and CCL2 levels. Serum samples from Cx3cr1−/−, Ccr2−/−, and Cx3cr1−/−Ccr2−/− double-knockout mice were assayed by ELISA for the presence of CX3CL1 and CCL2, and results are shown in the left y-axis (open symbols) and right y-axis (filled symbols), respectively. Bars show mean value with 95% confidence interval (CI) of chemokine in pg/mg of protein. Each point represents an individual mouse.
Circulating cells expressing wild-type receptors are sufficient to restore chemokine homeostasis
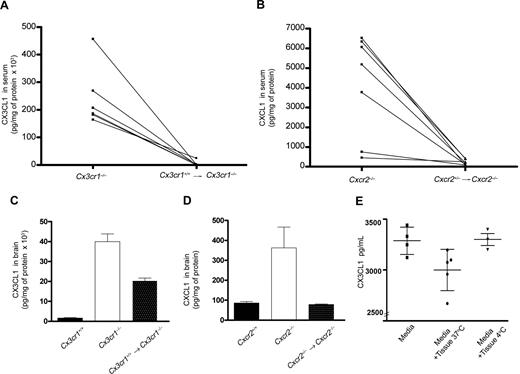
To determine whether receptor expression in circulating cells was sufficient to remove ligand from serum or tissues, we generated bone marrow radiation chimerae using lethally irradiated Cx3cr1−/− or Cxcr2−/− mice as recipients. Six weeks after bone marrow transfer, blood of chimeric mice was genotyped by PCR. For both WT → Cx3cr1−/− and HET → Cxcr2−/− bone marrow transfers, the presence of the wild-type allele in circulating cells (Figure S1) was indicative of successful chimerism. Analyses of serum levels of CX3CL1 and CXCL1 in WT → Cx3cr1−/− (Figure 2A) and WT → Cxcr2−/− (Figure 2B) chimeric mice, respectively, revealed a significant effect. Bone marrow reconstitution with wild-type cells reduced serum chemokines to basal levels. These results showed that reconstitution with wild-type bone marrow cells was enough to clear excess ligand from the peripheral circulation. A partial effect was observed in the brain of WT → Cx3cr1−/− mice (Figure 2C) that exhibited approximately half the amount of CX3CL1 detected in Cx3cr1−/− mice. This result is due to the low turnover rate of recipient parenchymal microglia that lack CX3CR1 expression. The presence of bone marrow–derived wild-type perivascular macrophages and parenchymal microglia in WT → Cx3cr1−/− mice accounts for the partial clearance of excess ligand in the brain. Reduction of CXCL1 in the brain of chimeric HET → Cxcr2−/− mice suggested that bone marrow–derived cells expressing CXCR2 have the capacity to reduce the high chemokine levels in the Cxcr2−/− brain.
Reconstitution with wild-type bone marrow was sufficient to clear excess ligand. Serum levels of CX3CL1 (A) and CXCL1 (B) were measured by ELISA before and 6 weeks after bone marrow transfer. The results reveal that reconstitution with wild-type cells significantly decreased serum levels of ligands to levels comparable with wild-type mice. Similarly, measurement of chemokines in CNS tissue of mice shows that CX3CL1 (C) and CXCL1 (D) were reduced 6 weeks after reconstitution with wild-type bone marrow. Clearance of CX3CL1 by CX3CR1 was evaluated in an in vitro brain slice preparation (E). Bars show mean value with 95% CI of CX3CL1 in pg/mL. Each point represents a value from individual wells in 2 independent experiments.
Reconstitution with wild-type bone marrow was sufficient to clear excess ligand. Serum levels of CX3CL1 (A) and CXCL1 (B) were measured by ELISA before and 6 weeks after bone marrow transfer. The results reveal that reconstitution with wild-type cells significantly decreased serum levels of ligands to levels comparable with wild-type mice. Similarly, measurement of chemokines in CNS tissue of mice shows that CX3CL1 (C) and CXCL1 (D) were reduced 6 weeks after reconstitution with wild-type bone marrow. Clearance of CX3CL1 by CX3CR1 was evaluated in an in vitro brain slice preparation (E). Bars show mean value with 95% CI of CX3CL1 in pg/mL. Each point represents a value from individual wells in 2 independent experiments.
To evaluate the effect of total body radiation on chemokine levels, we determined the levels of CX3CL1 and CXCL1 in irradiated wild-type recipients that were reconstituted with wild-type bone marrow (Cx3cr1+/+ → Cx3cr1+/+ and Cxcr2+/− → Cxcr2+/+). Serum CX3CL1 (520 ± 118 pg/mg protein, n = 6 mice) in Cx3cr1+/+ → Cx3cr1+/+ mice and CXCL1 (207 ± 53 pg/mg protein, n = 4) levels in Cxcr2+/− → Cxcr2+/+ chimeric control were comparable (P > .1) with what was found in unmanipulated wild-type mice (Figure 1; Tables 1,2). Moreover, Cx3cr1−/− → Cx3cr1−/− controls showed CX3CL1 levels (229.4 ng/mg protein, n = 3 mice) that were similar to those found in nonirradiated Cx3cr1−/− mice (Table 1). We did not evaluate the levels of CXCL1 in Cxcr2−/− → Cxcr2−/− controls, as Cxcr2−/− recipients are innately immunocompromised and radiation treatment further compromises their survival. These data exclude radiation chimerism as a determinant of chemokine levels and show that reduction of ligand levels in chimeric chemokine receptor–deficient recipient mice is attributed to their reconstitution with receptor-expressing cells.
To further support these findings, we performed a clearance experiment in which brain slices were incubated with mouse recombinant CX3CL1. We determined the amount of chemokine removed from the media, after one hour of incubation. Pilot experiments with slices from wild-type mice showed that levels of CX3CL1 were dramatically elevated after a brief incubation, because neurons release fractalkine after stress (data not shown). Therefore, we used tissue slices from Cx3cl1−/− mice with functional CX3CR1 to avoid the confound of ligand produced by the tissues. The results indicated that CX3CR1-expressing tissues (24 ± 0.7 mm3) remove an average of 300 pg ligand at 37°C (12 pg/mm3 tissue) after 1 hour in culture (P = .04) compared with incubation at 4°C (Figure 2E).
Effect of high levels of circulating CCR2 ligands on alternate receptors
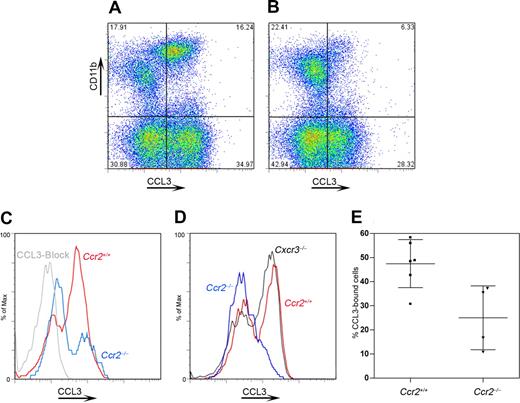
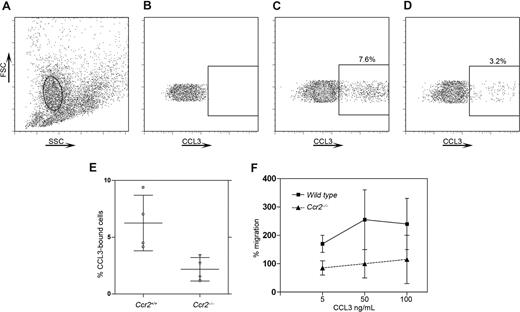
Given the complexity of chemokine/chemokine receptor biology and the ability of multiple ligands to signal via 2 or more receptors, we investigated the effect of excess CCR2 ligands (CCL2, CCL7, CCL8, CCL13) on the surface expression of other chemokine receptors such as CCR1 or CCR5. Specifically, we tested the hypothesis that excess CCR2 ligands might down-regulate other chemokine receptors. Using a binding assay for CCL3 (Figures 3,4), which is a CCR1 and CCR5 ligand, we found that in peripheral blood compared with wild-type mice, (Figure 3A) Ccr2−/− mice (Figure 3B) exhibited a significantly lower proportion of CCL3-bound CD11b+ cells (Figure 3B,C,E, P = .03 when comparing the proportion of Ccr2+/+ vs Ccr2−/− CD11b+ cells with bound CCL3). CCL3 binding was also analyzed in CX3CL1-deficient (data not shown) and CXCR3-deficient (Figure 3D) mice, where levels of binding were comparable with wild-type mice (Figure 3D), suggesting a specific defect in mice lacking CCR2. The CCL3-binding deficit in Ccr2−/− mice might be caused by lower CCR1 expression on circulating cells, which are impoverished for “inflammatory Ly6Chi” monocytes. Therefore, we also showed that binding of CCL3 was decreased in Ccr2−/− resident peritoneal cells (Figure 4A-E, P = .02) compared with wild-type peritoneal cells. To further investigate the biologic significance of our previous findings, we performed an in vitro chemotaxis assay using resident peritoneal cells and mouse recombinant CCL3 or CXCL12 (Figure 4F) in the bottom well. Wild-type cells but not Ccr2−/− cells showed a response to CCL3 (Figure 4F). However, responses to CXCL12 were not different between wild-type and Ccr2−/− resident peritoneal cells (data not shown). This result indicated that excess CCR2 ligands (some of which are also CCR1 or CCR5 ligands) are able to down-regulate other receptors.
Impaired CCL3 binding in CCR2-deficient PBMCs. Peripheral blood mononuclear cells were assayed for CCL3 binding as a measurement of CCR1/CCR5 function by flow cytometry. When comparing wild-type (A) and Ccr2−/− (B) mice, a decreased percentage of CD11b+ cells shows CCL3 binding in cells lacking CCR2; a specificity control using a CCL3-blocking antibody is shown (C). Decreased CCL3 binding was not observed in other chemokine receptor–deficient lines with intact CCR2 expression, such as Cxcr3−/− mice (D). Graphed results (E) revealed a 50% reduction in CCL3 binding in cells lacking CCR2. Bars show mean value with SD. Each point in the bar represents an individual mouse.
Impaired CCL3 binding in CCR2-deficient PBMCs. Peripheral blood mononuclear cells were assayed for CCL3 binding as a measurement of CCR1/CCR5 function by flow cytometry. When comparing wild-type (A) and Ccr2−/− (B) mice, a decreased percentage of CD11b+ cells shows CCL3 binding in cells lacking CCR2; a specificity control using a CCL3-blocking antibody is shown (C). Decreased CCL3 binding was not observed in other chemokine receptor–deficient lines with intact CCR2 expression, such as Cxcr3−/− mice (D). Graphed results (E) revealed a 50% reduction in CCL3 binding in cells lacking CCR2. Bars show mean value with SD. Each point in the bar represents an individual mouse.
Decreased CCL3 binding and CCL3-induced biologic responses in CCR2-deficient resident peritoneal cells. Resident peritoneal cells were assayed for CCL3 binding as described and the macrophage population was gated (A; solid oval). Plots of an unstained sample (B), wild-type cells (C), and Ccr2−/− cells (D) are shown. Similar to the previous results, a higher number of CCL3-bound cells was found in wild-type cells (C) compared with Ccr2−/− resident peritoneal cells (D,E). Ccr2−/− cells also exhibited a defective migratory response to CCL3 (F) but not to CXCL12 (not shown). Bars show mean value with SD in panels E and F. Each point in panel E represents an individual mouse. Each point in panel F represents the mean of 2 independent experiments.
Decreased CCL3 binding and CCL3-induced biologic responses in CCR2-deficient resident peritoneal cells. Resident peritoneal cells were assayed for CCL3 binding as described and the macrophage population was gated (A; solid oval). Plots of an unstained sample (B), wild-type cells (C), and Ccr2−/− cells (D) are shown. Similar to the previous results, a higher number of CCL3-bound cells was found in wild-type cells (C) compared with Ccr2−/− resident peritoneal cells (D,E). Ccr2−/− cells also exhibited a defective migratory response to CCL3 (F) but not to CXCL12 (not shown). Bars show mean value with SD in panels E and F. Each point in panel E represents an individual mouse. Each point in panel F represents the mean of 2 independent experiments.
Discussion
Chemokines, and their interaction with chemokine receptors, comprise a highly complex communication system that allows targeted function of circulating immune cells.18,19 The chemokine system also establishes a connection between CNS and peripheral elements during normal and pathological conditions.14 Biologic functions mediated by chemokine receptors require ligand binding and activation of receptor associated heterotrimeric G proteins. Various effector proteins are triggered by these G proteins leading to specific cellular responses including chemotaxis, increased respiratory burst, phagocytosis, and others.
Our results demonstrate a general role for the signaling chemokine receptors in chemokine clearance and homeostasis, which complements the well-characterized functions of the nonsignaling receptors,20,21 Duffy antigen receptor for chemokines (DARC),22-24 D6,25-27 and CCX-CKR (also known as CCRL1).28 DARC is abundant in erythrocytes and postcapillary venules, and erythrocyte DARC serves as a sink for the clearance of chemokines present at high levels in blood.23 D6, present on afferent lymphatic vessels, lymph nodes, and leukocytes,29 binds 12 inflammatory chemokines.26,30 Strong evidence implicates D6 in clearance of chemokines from inflamed skin.31 In addition, D6-deficient mice exhibited an increased susceptibility to the development of skin cancer,32 and D6 plays an important role in controlling tissue inflammation by acting as a chemokine scavenger on lymphatic vessels,30,33 leukocytes, and placenta.34 In contrast to D6, CCX-CKR28,35 is involved in the clearance of constitutive chemokines such as CCL19 and CCL21.24,36,37
Biologic functions of chemokines can be suppressed by silencing cognate chemokine receptors via 2 mechanisms.38 One involves chemokine receptor desensitization39 caused by steric hindrance of G-protein activation due to receptor phosphorylation by G-protein–coupled receptor kinases. The second comprises receptor down-regulation caused by receptor internalization,40,41 which occurs after ligand binds to the receptor. Depending on the extent of receptor expression per cell, this process may dramatically reduce the level of membrane expression of the receptor and therefore alter functionality.
To examine the roles of chemokine receptors in ligand consumption and in vivo clearance, we analyzed the levels of tissue and circulating ligand in various chemokine receptor–deficient mouse lines including members from the CC, CXC, and CX3C. These receptor-deficient lines allowed us to determine the levels of constitutive ligands such as CX3CL1, and the inflammatory ligands CCL2, CCL3, CXCL1, and CXCL10 in circulation (serum) and in brain during healthy conditions. Our data shows that CNS tissues of healthy Cx3cr1−/− and Cxcr2−/− mice have abnormally high (30-fold increase) levels of soluble CX3CL1 and CXCL1, respectively, compared with wild-type mice. Remarkably high levels of circulating CX3CL1, on the order of 300-fold excess, were detected in Cx3cr1−/− mice. CXCL1 and CXCL2 levels in the serum of Cxcr2−/− mice manifested a significant increase compared with normal wild-type littermates. CXCL10 levels in brain and serum were also significantly higher in Cxcr3−/− mice after induction of EAE compared with wild-type controls. Complementary results were obtained from analyses of serum levels of CCL2 and CX3CL1 in Ccr2−/− and Cx3cr1−/−Ccr2−/− double-knockout mice. Importantly, all serum chemokine elevations were corrected by generating radiation bone marrow chimerae, showing directly that the presence of chemokine receptor–positive circulating cells is sufficient to clear excess chemokine from the peripheral circulation. In contrast to the chemokine receptors that bind only one ligand, promiscuous receptors may partially compensate and clear excess chemokine levels, but at the same time alter their signaling cascade and decreasing functional expression.
There was no excess CX3CL1 mRNA in CNS tissues of Cx3cr1−/− mice, suggesting that failure to clear—rather than lack of transcriptional feedback inhibition—is causative for the chemokine elevations. Furthermore, we hypothesized that in the absence of CCR2 the increased levels of CCL2 and other ligands such as CCL7 or CCL8 down-regulate alternate receptors. Due to the limitation in reagents for staining CCR1 or detecting CCR2 ligands other than CCL2, we analyzed the presence of CCR1 in wild-type and CCR2-deficient mouse cells by performing CCL3-binding experiments and migration assays. The results supported the hypothesis; high levels of specific circulating chemokines that can signal to more than one receptor could affect the availability of alternate receptors.
Evidence of chemokine receptors in specific ligand homeostasis comes from early studies showing rapid utilization of CCL2 by wild-type macrophages, and increased levels of CCL2 in CCR2-deficient mice in response to alloantigen.12 Furthermore, a recent report showed that in patients affected by Sjögren syndrome (SS), an autoimmune disease characterized by infiltration of activated T cells around salivary gland ducts,42 CXCR3 behaves as a chemokine-scavenging receptor, and its role in SS cells is functionally impaired.43,44 The authors speculate that the impairment of this scavenging function might favor chemotaxis, leading to heightened immigration of CXCR3-positive T lymphocytes.44 In addition, it was reported that in a peritonitis model, Ccr5−/− mice had increased amounts of CCL3 and CCL5 in peritoneal exudates compared with wild-type mice, and it was established that CCR5 present on polymorphonuclear cells sequestered and effectively cleared CCL3 and CCL5 in vivo.45 In addition to these data, our results also suggest that signaling chemokine receptors are implicated in ligand homeostasis, however the molecular mechanism that couples signaling chemokine receptors and decoy receptor to their scavenging functions needs to be determined.
We have shown that elevated chemokine levels are present in vivo in chemokine receptor knockout strains. High levels of circulation ligands may produce effects by signaling to lesser-affinity receptors. An example is that CCR2 ligands, in high enough concentrations (CCL7, CCL8, CCL13), could signal to CCR1. CCL5/RANTES, if elevated in CCR5 knockouts, could signal to CCR1 or CCR3.46-48 In addition, this phenomenon needs to be taken into account as a potential regulatory mechanism during inflammatory conditions. Although chemokine elevation may represent a valuable biomarker for the efficacy of blocking molecules, observations will be pertinent for the potential use of chemokine receptor blockade for therapeutic purposes where unexpected (although not necessary detrimental) consequences might arise. The beneficial or deleterious consequences of high levels of circulating chemokine in patients exposed to chemokine receptor antagonist therapies should be carefully evaluated. Blocking chemokine receptors in human patients might produce analogous effects to chemokine receptor gene targeting in mice, with outcomes that would be unpredictable without taking these concerns into consideration.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Dr Cornelia Bergmann and Cathy Shemo for technical assistance with flow cytometry, and Dr Maria Febbraio and Difernando Vanegas for assistance with the establishment of the injection protocol for bone marrow transfers.
This work was supported by the National Institutes of Health (NS32151 [R.M.R.]) and the National Multiple Sclerosis Society (RG 3980-A-5 [R.M.R.] and TA 3021-A-1 [A.E.C.]).
National Institutes of Health
Authorship
Contribution: A.E.C. designed and performed the research, analyzed the data, and wrote the paper; R.M.R. designed the research, analyzed the data, and contributed to preparation of the paper; M.E.S., S.M.C., and M.M. provided technical support with the mouse colony and ELISA; S.M.C. performed qRT-PCR; L.L. and T.H. provided technical assistance with Cxcr2−/− tissues; C.S. provided analytical tools in flow cytometric studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard M. Ransohoff, Neuroinflammation Research Center, Department of Neurosciences, Lerner Research Institute, NC30, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: ransohr@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal