Abstract
The European Common Variable Immunodeficiency Disorders registry was started in 1996 to define distinct clinical phenotypes and determine overlap within individual patients. A total of 7 centers contributed patient data, resulting in the largest cohort yet reported. Patients (334), validated for the diagnosis, were followed for an average of 25.6 years (9461 patient-years). Data were used to define 5 distinct clinical phenotypes: no complications, autoimmunity, polyclonal lymphocytic infiltration, enteropathy, and lymphoid malignancy. A total of 83% of patients had only one of these phenotypes. Analysis of mortality showed a considerable reduction in the last 15 years and that different phenotypes were associated with different survival times. Types of complications and clinical phenotypes varied significantly between countries, indicating the need for large, international registries. Ages at onset of symptoms and diagnosis were shown to have a Gaussian distribution, but were not useful predictors of phenotype. The only clinical predictor was polyclonal lymphocytic infiltration, which was associated with a 5-fold increased risk of lymphoid malignancy. There was widespread variation in the levels of serum immunoglobulin isotypes as well as in the percentages and absolute numbers of B cells, confirming the heterogeneity of these conditions. Higher serum IgM and lower circulating CD8 proportions were found to be predictive markers for polyclonal lymphocytic infiltration and autoimmunity, respectively.
Introduction
Common variable immunodeficiency disorders (CVIDs) form a group of disorders of primary antibody production failure.1 These intrinsic diseases are the most common forms of clinical significant primary antibody failure in adults and children.
Less than 10% of these conditions are inherited.2 In some of these families, possible loci for dominant CVID genes on chromosomes 4q and 16q by genetic linkage studies have been identified. There is also rare evidence of recessive diseases involving B-cell genes (CD19)3 and T-cell genes (ICOS)4 in patients with these types of primary antibody failures. In addition, some polymorphisms in genes needed for B-cell survival (TACI and Msh5) are thought to be associated with CVIDs, due to a higher gene prevalence in patients compared with the general population.5,6
Diagnosis of a CVID is made by exclusion of currently known disorders of B-cell failure, including defects of B-cell differentiation (absent B cells), B-cell function (such as activation-induced cytidine deaminase [AID] and uracil-DNA glycosylase [UNG] deficiencies), and T-cell switching pathways, as well as those defects associated with distinct clinical or laboratory features. B-cell differentiation and class-switch disorders were avoided in this study by considering only patients who presented older than 4 years, those without a positive family history of infections in males,7 and those with an absence of previous opportunistic infections.
Even once these conditions have been excluded, it has been clear for some time that CVIDs represent many different conditions.8,9 Evidence for this includes the widely different clinical features of patients; the growing appreciation of the significance of normal or raised levels of IgM to indicate autosomal forms of hyper-IgM syndromes;10 phenotyping of lymphocyte T-, B-, and natural killer (NK)–cell subpopulations11 ; immunization responses;12 and the variable levels of B memory cells.13-17 Many papers in the last 2 decades have studied potential immunopathogenic mechanisms without reference to this heterogeneity, and this has resulted in a plethora of possible mechanisms in varying proportions of patients with clinically diverse CVIDs.
The hunt for genes relating to etiology or disease modification continues. Several genetic abnormalities in different immune pathways may account for the many distinct mechanistic failures in this group of late-onset but intrinsic antibody deficiencies. Before significant progress can be made, it may be helpful to define distinct clinical phenotypes more precisely and to investigate the extent of overlap of these phenotypes, as in other multifactorial diseases such as inflammatory bowel diseases. Moreover, it is important to distinguish those complications that are part of the underlying immune dysregulation from those that result from infections (such as bronchiectasis) in order to exclude the latter from criteria for disease phenotyping.
The CVID registry was established in 1996, with a grant from the European Union (EU) 5th Framework, to provide clinical data on these disorders in a large cohort of patients from different European countries. The aims of this first multicenter clinical data collection were as follows: (1) to define particular clinical phenotypes and to look for overlap within individual patients to lay a basis for correlations with genetic markers; and (2) to show any variation in the proportions of clinical phenotypes and individual complications between countries across Europe. There is some evidence that mortality of these patients has decreased,2,18,19 so it was important to examine the variable clinical phenotypes for survival to provide prognoses for particular clinical groups.
Methods
This registry was started in 1996 in Stockholm. At first 4 centers, 2 in Sweden (Stockholm and Gothenberg) and 2 in the United Kingdom (Oxford and the Royal Free Hospital, London), contributed patient data. A total of 3 other centers were added in 2002: Freiburg, Brno, and Paris. A range of clinical, immunologic, and follow-up anonymous data were collected in specific fields. This was checked, validated, and finally completed by request in 2005 and 2006. To ensure complete data collection, 3 meetings were held in 10 years, and there were 3 electronic reminders. The European Society for Immunodeficiencies (ESID) online register has now superseded this registry. Informed consent was not required in all countries in 1996, but patients were informed that data were being collected as part of clinical governance.
Validation of diagnosis
Standardized diagnostic criteria were used, consistent with the ESID/Pan- American Group for Immunodeficiency (PAGID) criteria20 : recurrent bacterial infections, age older than 4 years, serum IgG level below the lower normal range with at least one other serum immunoglobulin isotype (usually IgA) below lower limit of normal, and exclusion of an underlying cause. If no initial immunoglobulin levels were available, an individual was included provided that posttreatment levels of IgA or IgM met the criteria and there was evidence of a low IgG, usually in association with a low replacement dose or a break in therapy. Normal ranges for IgG levels varied between centers, so lower normal limits (fifth centile) was used. All patients had excessive infections and were on treatment.
Those with B cells less than 1% were included provided that X-linked agammaglobulinemia (XLA) had been excluded (BTK mutation/protein analysis, female sex, or older than 10 years at presentation). Other B-cell differentiation defects (λ5, μ, or BLNK) were considered to be rare in the absence of a family history. A total of 50 patients (31 female) had normal IgM levels; although these might represent autosomal-recessive hyper-IgM syndromes, they are included because they met the current CVID criteria.
If there were insufficient data to validate the diagnosis, all the data for that patient were excluded (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Where data for a particular section were incomplete, this was not included in the relevant analysis. The following numbers of patients were used: age at onset of diagnosis, 413; age at onset of symptoms, 389; diagnostic delay, 388; mortality, 352; and clinical phenotyping, 334.
Disease complications
Complications were entered into specific fields; additional or confirmatory data were obtained from pathology, imaging, and clinical problems sections to enable validation. Patients were included in this part of the analysis if they had been followed for at least 5 years since the onset of symptoms. Breakthrough infections were not considered to be related to the underlying immune dysregulation, only the result of the consequent antibody deficiency.
In determining the prevalence of each complication, it was important to realize that an individual patient might have more than one complication, and that these prevalences might add up to more than 100%.
Development of criteria for clinical phenotyping
The initial criteria for inclusion of a patient into a specific group are given as follows.
Viral infections: persistent infection with enterovirus, hepatitis B virus (HBV), and hepatitis C virus (HCV). This group was excluded subsequently, as this study was designed to account for natural disease progression only.
Autoimmunity: rheumatoid arthritis, systemic lupus erythematosus, seronegative arthritis (meeting the American Rheumatism Association [ARA] criteria), Graves disease, primary thyroid failure, insulin-dependent diabetes mellitus, pernicious anemia, atrophic gastritis (biopsy proven with antral sparing), chronic autoimmune hemolytic anemia, chronic immune thrombocytopenia, persistent unexplained neutropenia, psoriasis, and vitiligo. Transient arthropathy was excluded.
Polyclonal lymphocytic infiltration: this included lymphoid interstitial pneumonitis (LIP), unexplained granuloma (biopsy-proven; Crohn disease excluded), unexplained hepatomegaly (clinical examination or ultrasound), splenomegaly (on palpation or 11 cm or greater on ultrasound), and extensive and persistent lymphadenopathy (on palpation, ultrasound, or computed tomography [CT] scan).
Enteropathy: biopsy-proven lymphocytic infiltration in lamina propria and interepithelial mucous with villous atrophy, insensitive to gluten withdrawal.
Malignancies: lymphoid and other forms of cancer.
Structural damage: bronchiectasis (CT-proven); chronic sinusitis was not included due to lack of objective evidence.
No complications.
These criteria were later simplified on the basis of significant associations between particular complications and the exclusion of the commonest complications, namely bronchiectasis, splenomegaly and iron deficiency. Concurrent but unrelated conditions were not included.
Age of onset of significant symptoms and diagnostic delay
Age of onset was given for 384 of 389 patients; a decision was taken to define childhood as less than 13 years in 5 patients. Significant symptoms were defined as pneumonia, meningitis, or a significant increase of severity or frequency of bacterial infections in the respiratory tract.
Immunophenotyping of peripheral lymphocytes
In order to assess the value of T-, B-, and NK-lymphocyte percentages as predictors of clinical phenotype, analysis of pretreatment and posttreatment data for CD3+:CD4+, CD3+:CD8+, and CD19+ peripheral cells was done. A preanalysis check using serial data showed that using mixed before and after data did not result in significant differences. Examination of serial data on B cells on 177 patients from this registry show that there were no significant changes (≥ 15%) in peripheral CD19 B-cell numbers in 97% of the patients, as found previously (B. Ferry, N. Kaenzig, K. Packwood, J. Burden, D. Harrison, H.C., unpublished data, June 2006). Four year review of CVIDs patients in Oxford: is the classification by memory B-cell phenotypes reproducible? Unpublished data, June 1, 2006. Likewise, the figures for unchanging proportions of CD4+ and CD8+ cells were 92% and 90%, respectively.
Statistical analyses
For comparison of any 2 qualitative (categoric) variables, the calculation was based on the chi-square test for homogeneity using a Monte Carlo simulation of the exact distribution of the test statistic to determine the P value. This was needed because of the relatively small cell sizes in many of the contingency tables evaluated. Comparison of differences between centers used the Kruskal-Wallis nonparametric one-way analysis of variance (ANOVA) test of the median findings across the 7 centers.
The logistic regression model was used for evaluating the predictive effect of baseline laboratory results for the various phenotypes. Where the P value for a predictive effect of the given laboratory parameter was less than .05, then the odds ratio was given with a 95% confidence interval (CI). Note that the following transformations were used when needed in the analyses: IgG, square root; IgM, log; CD19, square root; CD19A, log; CD8, square root; CD4, square root; and CD4/CD8, log. These were identified from the Box-Cox family of data transformations.
Associations between the clinical phenotypes and age of onset of symptoms, age at diagnosis, and length of diagnostic delay were examined by the Mann-Whitney test. The Pearson correlation coefficient was used to examine the relationship between laboratory results. The aforementioned transformations were used in these analyses. For correlations between immunoglobulin levels and lymphocyte subpopulations, correlation coefficients were calculated for square root–transformed IgG levels and lymphocyte percentages and log-transformed absolute lymphocyte numbers.
The method of Kaplan and Meier was used to plot mortality data and estimate survival. Comparison of the survival distribution by phenotype was determined using the log-rank test.
Results
Validation of diagnosis and patient characteristics
Completion of the registry took 10 years. Data for 536 patients from 7 centers was checked to ensure that each individual met the diagnostic criteria for a CVID, though antibody responses were not available in all countries at diagnosis. A total of 112 patients were removed for the following reasons: inappropriate diagnosis (thymoma; 4 patients); secondary antibody defects (2 patients); normal serum levels of IgA and IgM (7 patients); IgG level within normal limits (11 patients); no serum immunoglobulin levels available (39 patients); no data on recurrent infections (36 patients), no data on infections or immunoglobulin levels (8 patients), and incomplete data for immunoglobulin levels (5 patients). The diagnosis was validated on 424 patients; patients with IgG subclass or specific antibody deficiency were excluded. In only 9 patients were IgG (3 patients) or IgA (6 patients) levels between 5.5 and 6.0 and 0.6 and 0.79 g/L (fifth to third centiles), respectively. A total of 64 (21%) patients had undetectable IgG, IgM, and IgA (IgG < 1.0g/L; IgA < 0.1 g/L; and IgM < 0.1 g/L). A total of 50 patients had IgM concentrations greater than 0.5 g/L (31 females; Figure S2). Particularly low levels of IgG at diagnosis (< 1.5 g/L) were not associated with diagnostic delay (P = .19) or severe infections (such as pneumonia or septicemia) before diagnosis (P = .84).
The ages of the patients at analysis ranged from 11 to 90 years, with a mean of 49.4 plus or minus 16.3 (mean and 1 SD). There were 177 males and 128 females (for 19 patients, sex was not specified).
The most common age at onset of symptoms was in the third decade (mean, 26.3 years; median, 24 years) and was continuous (Figure S3). There were no significant differences in the age at onset of symptoms between the country cohorts (P = .08). The age at diagnosis was recorded in 97% of patients (n = 413) and proved to be a continuous curve, though the rate of diagnosis fell in the eighth decade. The mean was 35.3 years, and the median was 33 years. More diagnoses were made in the United Kingdom under the age of 10 years (8%) when compared with Brno, Freiburg, and Sweden (2%; P = .002; Table S2).
Analysis of diagnostic delay (388 patients) showed a range of 0 to 61 years, with 20% of the patients being diagnosed with a CVID more than 15 years after the onset of symptoms. The mean diagnostic delay was 7.46 years, and median was 5 years, with 94.9% of the values being within 14.84 years (2 SD). There was an inverse correlation between the age of onset of symptoms and diagnostic delay (P < .001; Pearson correlation coefficient, −0.37). The hypothesis that a longer time span since the triggering event in these acquired diseases resulted in particularly low presenting serum IgG levels was not supported, since diagnostic delay did not correlate with IgG concentration at diagnosis (P = .19).
Length of follow-up and overall mortality
Times of follow-up since onset of symptoms, as well as time from diagnosis, were calculated for each patient. Data for age at onset of symptoms, and therefore length of follow-up, were available for 389 patients (Table S1). A total of 326 (84%) patients were followed for at least 10 years since onset of symptoms; 237 (61%) patients were followed for more than 18 years.
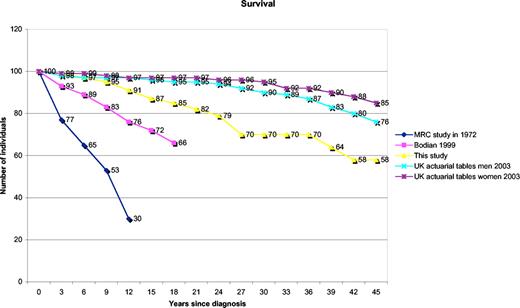
The mortality figures (Figure 1) are given relative to data from other cohorts published in 197118 and 1999,2 as well as figures from actuarial life tables for the United Kingdom.21 This shows that the 75th percentile for survival is 25 years after diagnosis (95% CI: 22-33 years) and the 60th percentile is 41 years (95% CI: 33-41 years). These figures do not account for the variable ages at presentation. However, the mean ages at onset of symptoms were significantly different in the surviving group (25 years) and the deceased group (34 years; P = .005). There were no associations between survival and sex (P = .78 by chi-square test) or initial serum IgG (P = .51), IgA (P = .19), or IgM (P = .06) levels.
Mortality by year since diagnosis. Patients in this study (yellow line) are compared with those reported previously in the relevant references. Kaplan-Meier plot of survival. Data for comparison from Cunningham-Rundles and Bodian2 (pink line), Healy et al18 (blue line), UK actuarial life figures 2003—men (turquoise line), and UK actuarial life figures 2003—women (purple line); because these are based on data from birth, they are both adjusted to start from the mean age of onset in patients, for comparison with start of patient data.
Mortality by year since diagnosis. Patients in this study (yellow line) are compared with those reported previously in the relevant references. Kaplan-Meier plot of survival. Data for comparison from Cunningham-Rundles and Bodian2 (pink line), Healy et al18 (blue line), UK actuarial life figures 2003—men (turquoise line), and UK actuarial life figures 2003—women (purple line); because these are based on data from birth, they are both adjusted to start from the mean age of onset in patients, for comparison with start of patient data.
A total of 51 patients died in this cohort; the median follow-up period (22.5 years) of those that died was not significantly different from that of survivors (22 years; P = .59). Causes of death were given for 37 (73%) patients. There were 10 deaths from disease-related complications: sepsis (4 patients), lymphoid malignancy (non-Hodgkin lymphoma; 5 patients), and unexplained liver disease (1 patient). A total of 9 deaths were due to complications of treatment: opportunistic infections (3 patients) and HCV infection (6 patients). The other deaths (18 patients), assumed to be unrelated, included cardiac/vascular diseases (11 patients), epithelial cancers (2 patients), and chronic obstructive airways disease related to smoking (5 patients).
From analysis of the overall data there was no relationship between mortality and the length of diagnostic delay (P = .33). Bronchiectasis was associated with reduced survival; 23 of 84 patients with bronchiectasis died, while only 28 of 250 without bronchiectasis died (P < .001).
Disease complications and comparison of complication rates between centers
The minimum considered for analysis of morbidity was 5 years; 19 patients who were followed for less than 5 years since onset of symptoms were excluded from the phenotyping analysis. Data from 64 patients from one center was excluded due to incompleteness. Low patient numbers (n = 7) from another center resulted in exclusion of that center to avoid selection bias. There was validated data regarding morbidity for 334 patients followed for more than 5 years, giving a total follow-up time of 9461 patient-years.
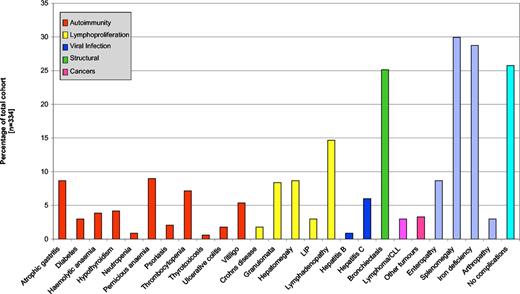
These 334 patients showed a wide range of individual complications (Figure 2). The prevalence of each complication is shown in Table 1. There was considerable variability in the prevalence of complications by center (Table 2; Figures S4A,B). The prevalence of splenomegaly varied from 3% to 66% (P < .001). Granuloma was also significantly different, with only 2% to 4% in the Swedish cohorts, 10% in Freiburg, and 16% in Oxford (P = .01). Unexplained hepatomegaly varied from 0% to 15% (P = .008), but differences in biopsy-proven enteropathy, although varying from 3% to 15%, was not significant (P = .06). Other differences between centers in the rates for specific complications included those for psoriasis, chronic immune thrombocytopenia, persistent lymphadenopathy, LIP, or unexplained iron deficiency. Pernicious anemia and autoimmune hemolytic anemia were not significantly variable (P = .08; Table 2).
Individual complications associated with CVIDs across Europe, as a percentage of the all patients. Patients may have had more than one complication.
Individual complications associated with CVIDs across Europe, as a percentage of the all patients. Patients may have had more than one complication.
Categories and prevalence of individual complications
| Category . | Prevalence, % . |
|---|---|
| Specific autoimmune diseases | |
| Cytopenias | |
| ITP | 7 |
| AHA | 4 |
| Neutropenias | 1 |
| Skin diseases | |
| Vitiligo | 5 |
| Psoriasis | 2 |
| Gastrointestinal diseases | |
| Pernicious anemia | 9 |
| Atrophic gastritis | 6 |
| Endocrine diseases | |
| Hypothyroidism | 4 |
| Thyrotoxicosis | 1 |
| Diabetes | 3 |
| Types of polyclonal lymphocytic infiltration | |
| Enteropathy* | 9 |
| Granuloma† | 8 |
| Unexplained hepatomegaly | 9 |
| Persistent lymphadenopathy | 15 |
| Splenomegaly | 30 |
| Lymphoid interstitial pneumonitis | 3 |
| Malignancies | 6 |
| Lymphoid malignancies | 3 |
| Other malignancies | 3 |
| No complications | 26 |
| Category . | Prevalence, % . |
|---|---|
| Specific autoimmune diseases | |
| Cytopenias | |
| ITP | 7 |
| AHA | 4 |
| Neutropenias | 1 |
| Skin diseases | |
| Vitiligo | 5 |
| Psoriasis | 2 |
| Gastrointestinal diseases | |
| Pernicious anemia | 9 |
| Atrophic gastritis | 6 |
| Endocrine diseases | |
| Hypothyroidism | 4 |
| Thyrotoxicosis | 1 |
| Diabetes | 3 |
| Types of polyclonal lymphocytic infiltration | |
| Enteropathy* | 9 |
| Granuloma† | 8 |
| Unexplained hepatomegaly | 9 |
| Persistent lymphadenopathy | 15 |
| Splenomegaly | 30 |
| Lymphoid interstitial pneumonitis | 3 |
| Malignancies | 6 |
| Lymphoid malignancies | 3 |
| Other malignancies | 3 |
| No complications | 26 |
Demonstration of the different types of autoimmune diseases, polyclonal lymphocytic infiltrative conditions, and malignancies in 334 patients, giving the prevalence of individual complications; any individual patient may have more than one complication.
Lymphocytic infiltration of small intestine that was not gluten-sensitive.
Biopsy-proven but regardless of site; excludes Crohn disease.
Differences in disease complication prevalences between centers
| Complication . | Prevalence by center, % . | P . | ||||
|---|---|---|---|---|---|---|
| Gothenberg . | Stockholm . | Freiburg . | Oxford . | Brno . | ||
| Iron deficiency | 22 | 4 | 18 | 56 | 29 | < .001 |
| Lymphadenopathy: persistent | 0 | 7 | 9 | 23 | 42 | < .001 |
| Splenomegaly | 3 | 19 | 46 | 30 | 66 | < .001 |
| Bronchiectasis | 14 | 4 | 16 | 52 | 27 | < .001 |
| No complications | 43 | 46 | 24 | 12 | 5 | < .001 |
| Lymphoid interstitial pneumonitis | 0 | 0 | 0 | 10 | 0 | < .001 |
| Hepatomegaly: unexplained | 0 | 5 | 13 | 15 | 7 | .008 |
| Granuloma: unexplained | 4 | 2 | 10 | 16 | 5 | .01 |
| Immune thrombocytopenia | 3 | 4 | 12 | 13 | 0 | .01 |
| Psoriasis | 0 | 2 | 0 | 6 | 0 | .02 |
| Enteropathy: unexplained | 3 | 4 | 10 | 13 | 15 | .06 |
| Pernicious anemia | 4 | 18 | 10 | 6 | 10 | .08 |
| Autoimmune hemolytic anemia | 1 | 4 | 3 | 8 | 0 | .08 |
| Thyrotoxicosis | 0 | 0 | 0 | 2 | 0 | .26 |
| Hypothyroidism | 0 | 4 | 6 | 5 | 7 | .28 |
| Persistent arthropathy | 1 | 2 | 6 | 2 | 5 | .46 |
| Neutropenia | 0 | 2 | 0 | 1 | 2 | .66 |
| Vitiligo | 6 | 5 | 6 | 5 | 5 | 1.0 |
| Complication . | Prevalence by center, % . | P . | ||||
|---|---|---|---|---|---|---|
| Gothenberg . | Stockholm . | Freiburg . | Oxford . | Brno . | ||
| Iron deficiency | 22 | 4 | 18 | 56 | 29 | < .001 |
| Lymphadenopathy: persistent | 0 | 7 | 9 | 23 | 42 | < .001 |
| Splenomegaly | 3 | 19 | 46 | 30 | 66 | < .001 |
| Bronchiectasis | 14 | 4 | 16 | 52 | 27 | < .001 |
| No complications | 43 | 46 | 24 | 12 | 5 | < .001 |
| Lymphoid interstitial pneumonitis | 0 | 0 | 0 | 10 | 0 | < .001 |
| Hepatomegaly: unexplained | 0 | 5 | 13 | 15 | 7 | .008 |
| Granuloma: unexplained | 4 | 2 | 10 | 16 | 5 | .01 |
| Immune thrombocytopenia | 3 | 4 | 12 | 13 | 0 | .01 |
| Psoriasis | 0 | 2 | 0 | 6 | 0 | .02 |
| Enteropathy: unexplained | 3 | 4 | 10 | 13 | 15 | .06 |
| Pernicious anemia | 4 | 18 | 10 | 6 | 10 | .08 |
| Autoimmune hemolytic anemia | 1 | 4 | 3 | 8 | 0 | .08 |
| Thyrotoxicosis | 0 | 0 | 0 | 2 | 0 | .26 |
| Hypothyroidism | 0 | 4 | 6 | 5 | 7 | .28 |
| Persistent arthropathy | 1 | 2 | 6 | 2 | 5 | .46 |
| Neutropenia | 0 | 2 | 0 | 1 | 2 | .66 |
| Vitiligo | 6 | 5 | 6 | 5 | 5 | 1.0 |
The P value provides the statistical evidence against the hypothesis of equal intercenter rates for each disease complication.
There were also different proportions of patients in each center with bronchiectasis (P < .001). Bronchiectasis was associated with previous serious infection (pneumonia/septicemia; P = .03), thus representing morbidity from serious pulmonary infection rather than from the underlying immune defect. There was no correlation with diagnostic delay (P = .8), time since onset of symptoms (P = .09), or smoking (P = .16), despite the finding that the proportion of patients who had never smoked varying from 11% in Brno to 44% in Freiburg.
Development of criteria for clinical phenotyping
The aim, to develop criteria for clinical phenotyping, depended on independence of complications from each other (Table 3). This led to a search for significant associations between the various complications. LIP was associated with granulomata (P < .001) and unexplained persistent lymphadenopathy (P < .001). Enteropathy was associated with iron deficiency (P < .001) but only weakly with unexplained hepatomegaly (P = .03) or lymphadenopathy (P = .01). There was also an association between iron deficiency and lymphadenopathy (P = .003).
Correlations between complications
| Complication . | Correlation, no. patients (P) . | ||||||
|---|---|---|---|---|---|---|---|
| Lymphoid interstitial pneumonitis . | Granuloma . | Enteropathy, unexplained . | Hepatomegaly, unexplained . | Lymphadenopathy, persistent . | Iron deficiency . | Splenomegaly . | |
| Lymphoid interstitial pneumonitis, n = 10 | — | 5 (< .001) | 1 (1.0) | 2 (.21) | 8 (< .001) | 6 (.04) | 7 (.009) |
| Granuloma, unexplained, n = 28 | — | 6 (.09) | 6 (.09) | 13 (< .001) | 13 (.047) | 17 (< .001) | |
| Enteropathy, unexplained, n = 29 | — | 6 (.03) | 9 (.01) | 17 (< .001) | 14 (.03) | ||
| Hepatomegaly, unexplained, n = 29 | — | 11 (.001) | 16 (.002) | 22 (< .001) | |||
| Lymphadenopathy, persistent, n = 49 | — | 23 (.003) | 35 (< .001) | ||||
| Iron deficiency, n = 96 | — | 36 (.06) | |||||
| Splenomegaly, n = 100 | — | ||||||
| Complication . | Correlation, no. patients (P) . | ||||||
|---|---|---|---|---|---|---|---|
| Lymphoid interstitial pneumonitis . | Granuloma . | Enteropathy, unexplained . | Hepatomegaly, unexplained . | Lymphadenopathy, persistent . | Iron deficiency . | Splenomegaly . | |
| Lymphoid interstitial pneumonitis, n = 10 | — | 5 (< .001) | 1 (1.0) | 2 (.21) | 8 (< .001) | 6 (.04) | 7 (.009) |
| Granuloma, unexplained, n = 28 | — | 6 (.09) | 6 (.09) | 13 (< .001) | 13 (.047) | 17 (< .001) | |
| Enteropathy, unexplained, n = 29 | — | 6 (.03) | 9 (.01) | 17 (< .001) | 14 (.03) | ||
| Hepatomegaly, unexplained, n = 29 | — | 11 (.001) | 16 (.002) | 22 (< .001) | |||
| Lymphadenopathy, persistent, n = 49 | — | 23 (.003) | 35 (< .001) | ||||
| Iron deficiency, n = 96 | — | 36 (.06) | |||||
| Splenomegaly, n = 100 | — | ||||||
— indicates not applicable.
Only intrinsically disease-related complications were used for clinical phenotyping. Complications were regrouped according to the results of the positive association analysis. The 5 clinical phenotyping categories were as follows: autoimmunity (including organ-specific autoimmune conditions and cytopenias); polyclonal lymphocytic infiltration (including unexplained granuloma, unexplained hepatomegaly, persistent lymphadenopathy, and LIP); lymphoid malignancy (proven and treated); unexplained enteropathy (biopsy-proven and gluten-insensitive); and no disease-related complications.
Bronchiectasis and splenomegaly were not used. Bronchiectasis was not related to the underlying disease (see “Disease complications and comparison of complication rates between centers”). Splenomegaly was too common and associated with many complications (Table 4), reflecting the wide variety of causes of splenic enlargement rather than a relationship to CVID pathogenesis. Iron deficiency, associated with most other complications, was also excluded. Transient arthropathy and Crohn disease were excluded due to uncertain etiology.
Role of splenomegaly
| . | With splenomegaly, no. patients . | No splenomegaly, no. patients . | P for positive association with splenomegaly . |
|---|---|---|---|
| Cytopenias alone | 21 | 12 | < .001 |
| Hepatomegaly | 22 | 7 | < .001 |
| Granuloma | 17 | 11 | < .001 |
| Enteropathy | 14 | 15 | .03 |
| Bronchiectasis | 33 | 51 | .04 |
| Cytopenias + solid organ autoimmunity | 4 | 3 | .2 |
| Solid organ–specific autoimmunity alone | 28 | 65 | 1.0 |
| Total | 100 | 234 |
| . | With splenomegaly, no. patients . | No splenomegaly, no. patients . | P for positive association with splenomegaly . |
|---|---|---|---|
| Cytopenias alone | 21 | 12 | < .001 |
| Hepatomegaly | 22 | 7 | < .001 |
| Granuloma | 17 | 11 | < .001 |
| Enteropathy | 14 | 15 | .03 |
| Bronchiectasis | 33 | 51 | .04 |
| Cytopenias + solid organ autoimmunity | 4 | 3 | .2 |
| Solid organ–specific autoimmunity alone | 28 | 65 | 1.0 |
| Total | 100 | 234 |
Numbers and types of complications in patients with and without splenomegaly and the level of significance for each.
Clinical phenotypes
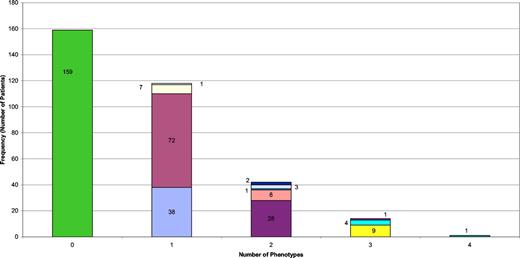
The 334 patients were divided into the 5 clinical phenotypes and absence of overlap was demonstrated in 83% of the cohort, while only 12.6% had criteria for 2 clinical phenotypes (Figure 3). Lymphoid malignancy occurred late in the progress of the disease and almost always in patients with preexisting polyclonal lymphocytic infiltration (7 of 10 patients; P = .007).
Nature and numbers of types of clinical phenotypes in individual patients. Green bar represent patients with only breakthrough infections (159 patients); all the others represent a noninfectious complication (with or without breakthrough infections). Blue bar indicates those with lymphocytic infiltration alone (38 patients); mauve bar, those with autoimmunity alone (72 patients); pale yellow bar, those with enteropathy alone (7 patients); pale blue bar, malignancy alone (1 patient); purple bar, lymphocytic infiltration and autoimmunity (28 patients); pink bar, enteropathy and lymphocytic infiltration (8 patients); blue bar, lymphocytic infiltration and malignancy (1 patient); pale purple bar, enteropathy and autoimmunity (3 patients); dark blue bar, autoimmunity and malignancy (2 patients); bright yellow bar, autoimmunity, lymphocytic infiltration, and enteropathy (9 patients); pale green bar, autoimmunity, lymphocytic infiltration, and malignancy (4 patients); dark red bar, lymphocytic infiltration, enteropathy, and malignancy (1 patient); dark green bar, autoimmunity, lymphocytic infiltration, enteropathy, and malignancy (1 patient).
Nature and numbers of types of clinical phenotypes in individual patients. Green bar represent patients with only breakthrough infections (159 patients); all the others represent a noninfectious complication (with or without breakthrough infections). Blue bar indicates those with lymphocytic infiltration alone (38 patients); mauve bar, those with autoimmunity alone (72 patients); pale yellow bar, those with enteropathy alone (7 patients); pale blue bar, malignancy alone (1 patient); purple bar, lymphocytic infiltration and autoimmunity (28 patients); pink bar, enteropathy and lymphocytic infiltration (8 patients); blue bar, lymphocytic infiltration and malignancy (1 patient); pale purple bar, enteropathy and autoimmunity (3 patients); dark blue bar, autoimmunity and malignancy (2 patients); bright yellow bar, autoimmunity, lymphocytic infiltration, and enteropathy (9 patients); pale green bar, autoimmunity, lymphocytic infiltration, and malignancy (4 patients); dark red bar, lymphocytic infiltration, enteropathy, and malignancy (1 patient); dark green bar, autoimmunity, lymphocytic infiltration, enteropathy, and malignancy (1 patient).
There were significant differences between countries for each of the clinical phenotypes (Table 5) as well as individual complications. The prevalence of polyclonal lymphocytic infiltration was markedly low in Sweden (P < .001). The prevalence of patients who had no complications was lowest in the Czech Republic (P < .001).
Clinical phenotypes: variation of prevalences between countries
| Phenotype . | Czech Republic, no. patients (%) . | Germany, no. patients (%) . | Sweden, no. patients (%) . | United Kingdom, no. patients (%) . | P . |
|---|---|---|---|---|---|
| Polyclonal lymphocytic infiltration | 22 (54) | 21 (31) | 15 (12) | 37 (39) | < .001 |
| Infections only | 14 (34) | 27 (40) | 79 (61) | 36 (37) | < .001 |
| Autoimmunity | 12 (29) | 26 (38) | 35 (27) | 46 (48) | .02 |
| Enteropathy | 6 (15) | 7 (10) | 4 (3) | 12 (13) | .03 |
| Lymphoid malignancy | 0 | 4 (6) | 1 (0.8) | 5 (5) | .07 |
| Total | 41 | 68 | 129 | 96 |
| Phenotype . | Czech Republic, no. patients (%) . | Germany, no. patients (%) . | Sweden, no. patients (%) . | United Kingdom, no. patients (%) . | P . |
|---|---|---|---|---|---|
| Polyclonal lymphocytic infiltration | 22 (54) | 21 (31) | 15 (12) | 37 (39) | < .001 |
| Infections only | 14 (34) | 27 (40) | 79 (61) | 36 (37) | < .001 |
| Autoimmunity | 12 (29) | 26 (38) | 35 (27) | 46 (48) | .02 |
| Enteropathy | 6 (15) | 7 (10) | 4 (3) | 12 (13) | .03 |
| Lymphoid malignancy | 0 | 4 (6) | 1 (0.8) | 5 (5) | .07 |
| Total | 41 | 68 | 129 | 96 |
Mortality and clinical phenotypes
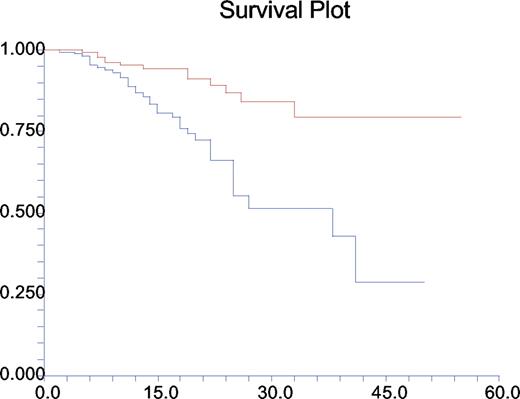
Once clinical phenotypes were assigned, patient survival was divided into 2 groups: those with disease-related phenotypes and those without. The resulting Kaplan-Meier plot (Figure 4) shows that there is a significant difference between these groups of patients (P < .001).
Mortality by years since diagnosis and by clinical phenotype. Red line represents those without complications; blue line, those with at least one disease-related complication. Kaplan-Meier plot of survival.
Mortality by years since diagnosis and by clinical phenotype. Red line represents those without complications; blue line, those with at least one disease-related complication. Kaplan-Meier plot of survival.
Further analysis by particular clinical phenotype showed that the highest mortality rates were for those patients with the enteropathy phenotype (P < .001) or the polyclonal lymphocytic infiltrative phenotype (P < .001); relative risks (RRs) were 4.0 and 3.0, respectively. Not surprisingly, there was an association between increased mortality and lymphoid malignancy (P = .002; RR, 5.5) and a lesser but significant association with autoimmunity (P = .03; RR, 2.5).
Predictive markers for clinical phenotypes
In order to determine predictive markers for clinical phenotypes, several parameters were examined. Neither autoimmunity nor polyclonal lymphocytic infiltration varied with sex (P ≥ .05). There were no significant differences in the ages at first symptoms for polyclonal lymphocytic infiltration (P = .12), enteropathy (P = .23), or malignancy (P = .64), though there were significant associations between later onset of symptoms with the “no complication” phenotype (P < .001) and also with autoimmunity (P = .01).
Age at diagnosis was also considered by comparing these ages for those with and without each particular phenotype. Those with no complications had been diagnosed with a CVID significantly later (P < .001) than those without, as were those with autoimmunity (P < .001). There were no associations between the presence of any particular clinical phenotype and the length of diagnostic delay.
Once the clinical phenotyping was complete, the data were reanalyzed to ascertain any differences in the prevalence of clinical phenotypes at 5, 10, or even 15 years since age of onset of symptoms. Logistic regression for time since onset of symptoms and clinical phenotype showed no significant differences in autoimmunity or polyclonal lymphocytic infiltration when analyzed by time since onset (P ≥ .05).
A search for correlations between presenting serum IgG, IgA, and IgM levels and clinical phenotypes revealed a significant correlation between serum IgM level and the eventual development of either polyclonal lymphocytic infiltration (P = .018) or a lymphoid malignancy (P = .02). For each additional 1 g/L of IgM (in the neighborhood of the median value of 0.18), there were 16% higher odds that the patient will develop polyclonal lymphocytic infiltration. In relation to lymphoid malignancy, there were 31% higher odds that the patient will have a lymphoid malignancy for each additional 1 g/L of IgM. In contrast, IgG did not predict this phenotype (P = .17).
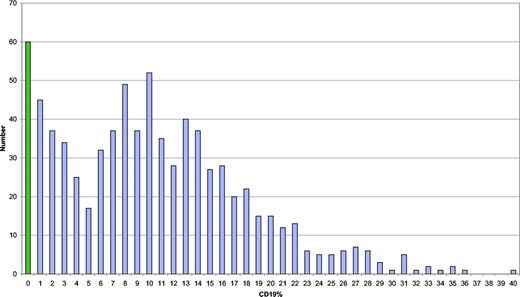
The data were analyzed for significant associations between lymphocyte marker percentages and clinical phenotypes. Although CD19 cell percentages (n = 285) had a bimodal, skewed distribution with 2 peaks, with a cutoff at 5% (Figure 5), there were no significant associations with clinical phenotypes. Of the 46 values showing apparently abnormally high proportions of B cells in the periphery (≥ 24%), 27 were associated with either polyclonal lymphocytic infiltration (14 of 46 [30%]) or autoimmunity (13 [28%]); only 1 was associated with a lymphoid malignancy. Baseline CD8 proportions were significantly associated with autoimmunity (P = .04); for each additional 10% higher CD8 level, there were 18% lower odds that the patient would have an autoimmune phenotype. There was an inverse correlation between IgG level at presentation and percentage of CD4 cells (Pearson correlation coefficient r = −.23; P < .001), but this was not the case for CD8 cells (r = .08; P = .24).
Distribution of B cells in the circulation (as a percentage). Number of values of B cells on the y-axis.
Distribution of B cells in the circulation (as a percentage). Number of values of B cells on the y-axis.
Discussion
Previous reports of cohorts of patients with CVIDs include data from 248 patients seen in one U.S. city over 20 years,2 and a report of 224 patients from centers throughout Italy, with a mean follow-up of 11.5 years (range, 3-36 years).19 This current report represents the largest cohort studied so far and involves patients from several European countries. CVIDs are heterogeneous, so studies can be confounded by variations between patients. The definition of CVID used here included evidence of recurrent or severe infections, in association with reduced serum immunoglobulin levels, in order to be rigorous. All patients were receiving replacement immunoglobulin therapy. Despite the wide range of IgG levels, most patients (94.2%) had initial IgG levels less than 4.5 g/L at diagnosis, suggesting that diagnostic criteria need revision.
The new finding that IgG levels less than 1.5 g/L were not associated with severe infections before diagnosis was reassuring. This is consistent with the report that pneumonia is associated rather with low numbers/percentages of B memory cells.17 The absence of a correlation between low IgG levels and length of diagnostic delay argues against gradual development of CVIDs; however, without serial immunoglobulin measurements as part of health screening, this is not testable.
The data show a continuum of ages at onset of symptoms as well as ages at diagnosis, rather than 2 peaks as reported previously.2 The range of complications in patients with CVIDs has been well described in a number of studies,2,22,32 with similar prevalences to this report. However, to date, there have been no studies to compare data from different countries. The rates of individual complications in Sweden were similar between the 2 centers but lower than in other countries. This difference was most marked in polyclonal lymphocytic infiltrative states, reflecting the variation between countries in rates of granuloma, persistent lymphadenopathy, LIP, and unexplained hepatomegaly. There was variation in autoimmunity in this study too, similar to findings in psoriasis and idiopathic thrombocytopenic purpura (ITP) documented previously.33,34 It is unlikely that these are due to different styles of management of patients with CVID, since this is uniform across Europe, particularly in large centers. These differences provide further evidence that CVIDs are polygenic diseases, with the variations reflecting different genetic backgrounds, and will inform the search for disease-causing and disease-modifying mutations in specific populations.
There were striking associations between complications, though these were suggested from clinical practice. These may indicate similar causes, though not necessarily common etiologic factors. The association of granulomata and LIP has been reported before.35 HHV8 was suggested as a cause of LIP, though the virus was not found consistently in biopsy specimens, suggesting that multiple causes were more likely.36 The significant relationship between gluten-resistant enteropathy and unexplained hepatomegaly suggests that the liver disease could be secondary to an unknown infiltrative process in the small bowel. The nature of liver disease in patients with CVID is under review currently (C. Ward, oral and written communication, February 2008); the association with persistent lymphadenopathy and enteropathy is intriguing.
The criteria for each phenotype were based on associations of complications in an attempt to separate the infiltrative conditions from autoimmunity and avoidance of common complications. Splenomegaly was associated with a wide range of complications, including cytopenias, hepatomegaly, and granulomata, and not with solid organ–specific autoimmunity; splenomegaly was therefore not used. Bronchiectasis was significantly related to prior serious infection and not part of the underlying process. The high prevalence of iron deficiency anemia (29%) was not uniquely related to enteropathy (37% of patients were women in their reproductive years). None of these common complications were used as criteria. Enteropathy was the only infiltrative complication that was not strongly associated with persistent lymphadenopathy and was taken as a distinct phenotype; it is possible that the cause of hepatomegaly might also be distinct, and this would bear examination in larger studies in the future.
Clinical phenotyping resulted in 83% of the patients exhibiting features of only one clinical phenotype, giving credence to the criteria selected. Furthermore, each distinct phenotype had a particular correlation with survival. The highest mortality rates were in those patients with enteropathy or polyclonal lymphocytic infiltration. The association between granulomata/LIP and shortened survival has been reported before.35 There was also an association between mortality and lymphoid malignancy (not surprisingly), and a lesser but significant association with autoimmunity. This data, and the Kaplan-Meier plot (Figure 4), give weight to the biological validity of the criteria for clinical phenotyping. However, this analysis is based on retrospective data and it is important that these findings be confirmed by prospective studies in several continents.
Comparison of overall survival data with previously published cohorts shows a considerable increase in survival in recent years (Figure 1).2,18,22 Comparison with data from Iran, a country in which postdiagnosis survival (14% at 14 years) was very poor prior to the availability of therapeutic immunoglobulin,37 suggests that such treatment is effective in prolonging life. Reduced diagnostic delay does not account for the improvement in survival, since this has changed little.2,38 The calculated mean diagnostic delay for 388 patients was 7.46 years, with a median of 5 years, consistent with the Italian figure of 8.9 years.19 Although the mean ages at onset of symptoms were significantly different in the surviving versus the deceased group, a larger prospective cohort is needed to examine survival against age at onset of symptoms.
An attempt was made to search for predictors for any given clinical phenotype. These included demographic data, ages of onset of symptoms and diagnosis, length of diagnostic delay, associations between the phenotypes, and laboratory parameters. Sex, time since onset of symptoms, and length of diagnostic delay were not associated with a particular clinical phenotype of CVID. The development of complications does not appear to be time dependent but rather intrinsic to the disease processes.
The hypothesis that a longer time span since a triggering event in these acquired diseases results in particularly low presenting serum IgG levels was not supported either, since diagnostic delay did not correlate with IgG concentration at diagnosis (P = .19). The association between autoimmunity and later age at diagnosis (P < .001) may indicate lack of awareness of this association and is unexpected, since autoimmunity is usually more common in young females. Those patients without complications had also been diagnosed with CVID later in life, in comparison with those with a more complicated clinical phenotype (P < .001). This confirms the reported experience that children with CVIDs have a high incidence of complicated disease.39
The only clinical predictor was polyclonal lymphocytic infiltration; those with this clinical phenotype had a 5-fold increased risk of lymphoid malignancy. This is consistent with a 2-step transformation, as for non-Hodgkin lymphomas.
In regard to laboratory parameters, initial serum IgG levels was not predictive, but there was a significant correlation between serum IgM level and the eventual development of either polyclonal lymphocytic infiltration (P = .018) or a lymphoid malignancy (P = .02). This is similar to class-switch recombination defects and suggests this type of gene defect may be relevant in the 50 patients with IgM concentrations greater than 0.5 g/L, a group in whom further search for a defect in T-cell involvement in immunoglobulin switching is indicated.10,40 The substantial proportion of patients with CVID (21%) with very low levels of all 3 isotypes suggests that differentiation defects are also important in some of these disorders, as does the finding of 2 peaks in the distribution of CD19 cells. Failure of B memory cells has been shown to be important in clinically significant primary antibody failure.17 Recently, B memory cell numbers and types have been used to provide a laboratory classification of CVIDs, but how this will relate to these precise clinical phenotypes remains uncertain.41
Baseline CD8 proportions showed a significant inverse correlation with autoimmunity. There were no other significant associations with percentages of CD19, CD4, and CD8 cells and any other clinical phenotypes, though FOXP3+ cells were not examined in this study.42 There was an inverse correlation between IgG level at presentation and percentage of CD4 cells but not CD8 cells.
The original analysis of those 334 well-documented patients who had been followed for at least 5 years from onset of symptoms (average, 25.6 years) formed the basis of clinical phenotyping. Later analysis showed no differences in the prevalences of any particular clinical phenotype at 5, 10, or even 15 years since age of onset, suggesting that the clinical phenotype can be determined 5 years after the onset of symptoms. This is not surprising, since up to 15% of patients present with complications.22 However, the time to stability of clinical phenotype needs confirmation by longitudinal studies before the shorter time interval can be used for prognosis, so the date of onset of complications must be documented in future registries.
In conclusion, clinical phenotyping has been shown to be possible and important for prognosis. There is no association between diagnostic delay and the clinical phenotype of an individual patient, possibly indicating that this is part of the disease rather than due to subsequent infection. Lymphoid malignancy is associated with preexisting polyclonal lymphocytic infiltration, and correlates with serum IgM at diagnosis (although not IgG). Very low serum IgG levels are not associated with increased numbers of severe infections prior to diagnosis. Autoimmunity is correlated with lower levels of CD8+ cells. These observations provide a basis for the development of more sophisticated prognostic indicators and will encourage further studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Siraj Misbah for useful discussions as well as Berne Ferry and Eduardo Lopes-Granados, who also read the manuscript. Benjamin Gattmann also helped to input data from Freiburg.
This work was supported by funding from the European Union 5th Framework (grant EUROPID QLQ1-CT-2001-01395 to H.C. and L.H.), European Union 6th Framework (grant EUROPOLICY SP23-CT-2005-006411 to H.C. and B.G.), Baxter Healthcare (H.C.), the UK Primary Immunodeficiency Association (H.C.), the Ministry of Health of the Czech Republic (grant no. NR9035-4 to V.T.), the German Research Foundation (DFG grant GR1617/3) and the European Commission Marie-Curie grant MEXT-CT-2006-042316 (B.G.), the Jeffrey Modell Foundation (L.H. and H.C.), and the Stockholm County Fund (L.H.).
Authorship
Contribution: H.C., J.B., L.H., and A.D.B.W. designed the study; M. Lucas and M.R.A. managed the database; M. Lucas, J.B., D.W., B.G., C.F., V.T., and M.R.A. performed data entry; H.C., M. Lucas, M. Lee, and V.T. performed database analysis; and H.C., M. Lucas, M. Lee, L.H., V.T., and B.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helen Chapel, Department of Clinical Immunology, Nuffield Department of Medicine, Level 4A, Academic Street, John Radcliffe Hospital, Oxford, United Kingdom; e-mail: helen.chapel@ndm.ox.ac.uk.