In this issue of Blood, Su and colleagues report that a myristolyated cell-permeable peptide, RGT, corresponding to the extreme carboxyl terminus of the integrin subunit β3, specifically inhibits αIIbβ3-dependent Src-mediated “outside-in” signaling in platelets.
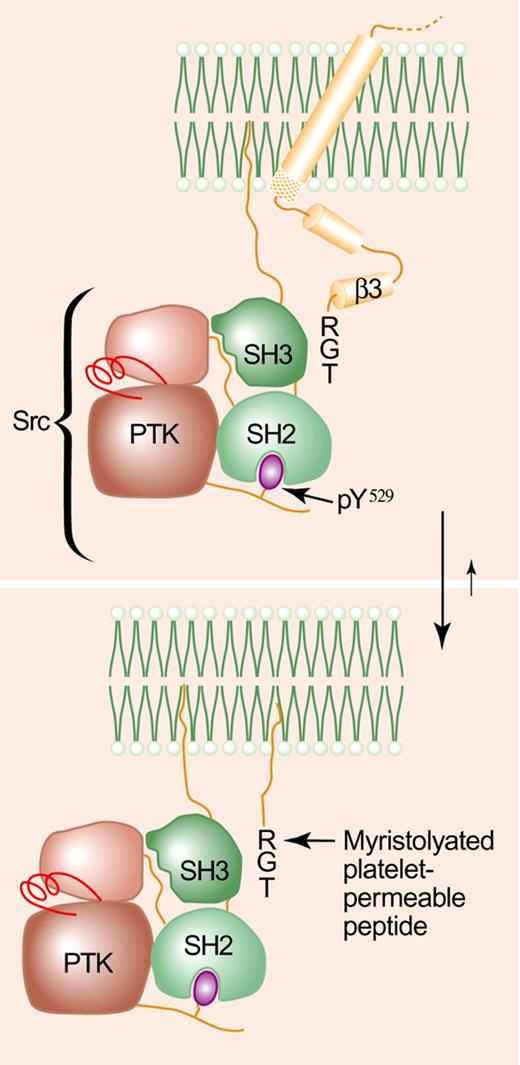
Depending on the strength of the stimulus, platelets undergo secondary aggregation (ie, the second wave of platelet aggregation), spreading, granule secretion, and support clot retraction as a result of αIIbβ3-dependent outside-in signaling. In turn, these αIIbβ3-dependent processes result from a cascade of tyrosine phosphorylation events that is initiated by the autophosphorylation and activation of the tyrosine kinase Src and/or other members of the Src family of tyrosine kinases.1 Src is highly expressed in platelets where approximately 3% of the enzyme is constitutively associated via its SH3 domain with the Arg-Gly-Thr (RGT) sequence located at the extreme carboxyl terminus of the β3 cytoplasmic tail.2 β3-associated Src is poised for catalysis, but it is stabilized in an inactive conformation, at least in part, by an intramolecular interaction between its SH2 domain and phosphorylated Tyr529 located in a carboxyl-terminal extension of its kinase domain.1 Phosphorylation of Tyr529 is maintained by the tyrosine kinase Csk, which is also associated with the β3 cytoplasmic tail.3 However, following fibrinogen binding to αIIbβ3, Csk dissociates from β3, coincident with the recruitment of the tyrosine phosphatase PTP-1B to the β3-Src complex.4 The resulting dephosphorylation of Tyr529 unclasps Src, enabling the autophosphorylation of Tyr418 and the activation of Src as the fibrinogen-occupied αIIbβ3 oligomerizes.1
The RGT-dependent association of Src with the β3 cytoplasmic tail is disrupted by a myristolyated RGT peptide. RGT indicates arginine-glycine-threonine; PTK, protein tyrosine kinase domain. The figure was adapted with permission from Li et al5 and Harrison.6 Professional illustration by Paulette Dennis.
The RGT-dependent association of Src with the β3 cytoplasmic tail is disrupted by a myristolyated RGT peptide. RGT indicates arginine-glycine-threonine; PTK, protein tyrosine kinase domain. The figure was adapted with permission from Li et al5 and Harrison.6 Professional illustration by Paulette Dennis.
On the basis of this paradigm, Su and colleagues hypothesized that a peptide corresponding to the carboxyl terminus of β3 would be expected to compete with platelet β3 for Src and to selectively inhibit αIIbβ3-mediated outside-in signaling. Moreover, they speculated that should this strategy for inhibiting secondary platelet responses prove to be successful, it could be used to inhibit platelet-mediated thrombosis. In this issue of Blood, Su et al report that in fact, a myristolyated RGT peptide does all that is asked of it. It translocates into the platelet interior, where it inhibits platelet adhesion and spreading on fibrinogen; it impairs platelet-mediated clot retraction; it inhibits second-wave, but not first-wave, platelet aggregation; and it impairs thrombin-stimulated P-selectin exposure. Importantly, in support of its proposed mechanism of action, the peptide also inhibits the Src-catalyzed phosphorylation of β3 tail residues Tyr747 and Tyr759; it binds to recombinant Src SH3 domains; and it dissociates Src, but not talin, from recombinant β3 cytoplasmic tails.
The results reported by Su et al are remarkable for several reasons. First, considering the large amount of Src in platelets, the small amount of Src associated with the β3 tail, and the relatively weak nature of the latter interaction, it is noteworthy that the myristolyated peptide essentially abrogated the interaction of Src with β3. Perhaps myristolyation increased the local concentration of peptide at the membrane, thereby enhancing its ability to compete with β3 for Src. Second, one might have predicted that because myristolyated RGT separated Src from Csk, it might have an activating, rather than an inhibiting, effect. The observation that the latter is the case suggests that β3, perhaps via αIIbβ3 clustering, also makes an essential contribution to the initiation of outside-in signaling. In this regard, it would also have been instructive to have observed the effect of the peptide on agonist-stimulated secretion by Glanzmann thrombasthenia platelets lacking αIIbβ3. Lastly, whether dissociating Src from the β3 cytoplasmic tail represents a viable therapeutic strategy is interesting to ponder, but it is unclear why it would be more efficacious than the currently available therapeutic options of aspirin and clopidogrel.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal