Abstract
Erythroblastic islands, the specialized niches in which erythroid precursors proliferate, differentiate, and enucleate, were first described 50 years ago by analysis of transmission electron micrographs of bone marrow. These hematopoietic subcompartments are composed of erythroblasts surrounding a central macrophage. A hiatus of several decades followed, during which the importance of erythroblastic islands remained unrecognized as erythroid progenitors were shown to possess an autonomous differentiation program with a capacity to complete terminal differentiation in vitro in the presence of erythropoietin but without macrophages. However, as the extent of proliferation, differentiation, and enucleation efficiency documented in vivo could not be recapitulated in vitro, a resurgence of interest in erythroid niches has emerged. We now have an increased molecular understanding of processes operating within erythroid niches, including cell-cell and cell-extracellular matrix adhesion, positive and negative regulatory feedback, and central macrophage function. These features of erythroblast islands represent important contributors to normal erythroid development, as well as altered erythropoiesis found in such diverse diseases as anemia of inflammation and chronic disease, myelodysplasia, thalassemia, and malarial anemia. Coupling of historical, current, and future insights will be essential to understand the tightly regulated production of red cells both in steady state and stress erythropoiesis.
Discovery
There is a great deal of recent excitement concerning stem cell niches that regulate self-renewal and cell differentiation in the bone marrow, but it should be noted that the first description of a hematopoietic niche actually took place 50 years ago when the French hematologist, Marcel Bessis, discovered erythroblastic islands.1 Cell-cell and cell–extracellular matrix interactions that are the hallmark of niches are increasingly being recognized as sites of both positive and negative regulators of cell proliferation and differentiation in many tissue types. As such, features of erythroid niches represent important contributors to normal erythroid development, as well as altered erythropoiesis found in such diverse diseases as thalassemia with its ineffective erythropoiesis, myelodysplasia (MDS) accompanied by disordered erythroid maturation, and anemia of inflammation and chronic disease secondary to perturbed iron metabolism.
Bessis originally characterized the erythroblastic island as developing erythroblasts surrounding a central macrophage, based on careful analysis of transmission electron micrographs of sections of bone marrow (Figure 1). Erythroid islands are also present in fetal liver and spleen.1 It is important to note that these islands are not seen in bone marrow aspirates because they are readily disrupted during smear preparation. Based on structural observations, Bessis and colleagues made a number of interesting inferences concerning the role of central macrophages. It was suggested that the macrophage functions as a “nurse” cell, providing iron to developing erythroblasts for heme synthesis.2 Furthermore, they theorized that extruded nuclei are phagocytosed by central macrophages at the termination of erythroid differentiation.1 These concepts proved prescient as they have been supported by a number of recent findings.
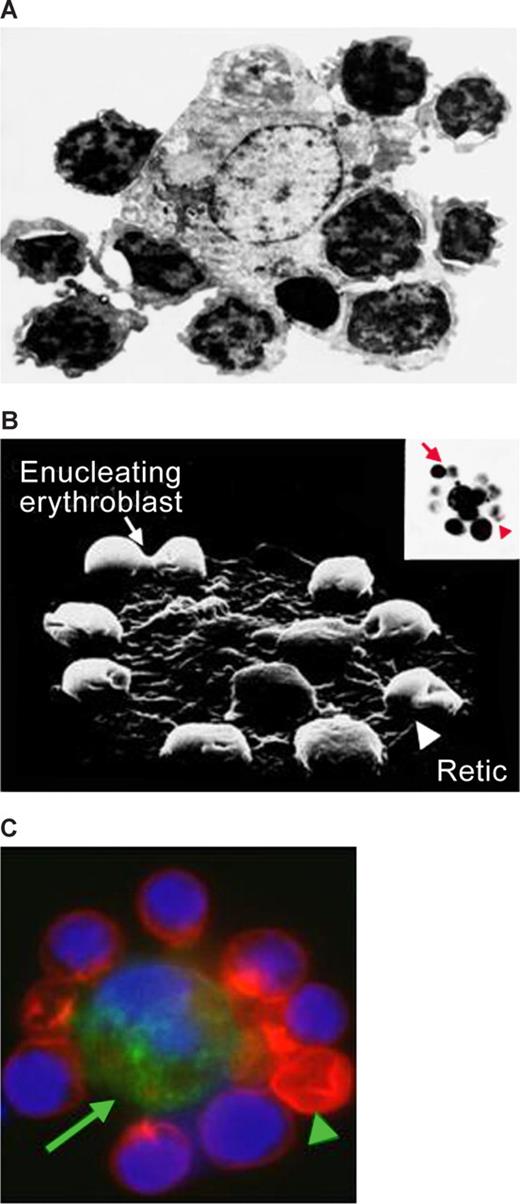
Micrographs of erythroblastic islands. (A) Transmission electron micrograph of an erythroblastic island isolated from rat bone marrow. Note the extensive cell-cell contact. (B) Scanning electron micrograph of an isolated erythroblastic island. The inset shows an optical microscopic image of the same structure. Note the presence of an enucleating erythroblast ( ) and a multilobulated reticulocyte (
) and a multilobulated reticulocyte ( ). (C) Confocal immunofluorescence image of an island reconstituted from freshly harvested mouse bone marrow cells stained with erythroid-specific marker (red), macrophage marker (green) and DNA probe (blue). Central macrophage is indicated by an arrow and a multilobulated reticulocyte by an arrowhead. (Panels A and B are courtesy of Michel Prenant of France, and panel C is reprinted from Lee et al24 with permission from the American Society of Hematology.) Illustration by Paulette Dennis.
). (C) Confocal immunofluorescence image of an island reconstituted from freshly harvested mouse bone marrow cells stained with erythroid-specific marker (red), macrophage marker (green) and DNA probe (blue). Central macrophage is indicated by an arrow and a multilobulated reticulocyte by an arrowhead. (Panels A and B are courtesy of Michel Prenant of France, and panel C is reprinted from Lee et al24 with permission from the American Society of Hematology.) Illustration by Paulette Dennis.
Micrographs of erythroblastic islands. (A) Transmission electron micrograph of an erythroblastic island isolated from rat bone marrow. Note the extensive cell-cell contact. (B) Scanning electron micrograph of an isolated erythroblastic island. The inset shows an optical microscopic image of the same structure. Note the presence of an enucleating erythroblast ( ) and a multilobulated reticulocyte (
) and a multilobulated reticulocyte ( ). (C) Confocal immunofluorescence image of an island reconstituted from freshly harvested mouse bone marrow cells stained with erythroid-specific marker (red), macrophage marker (green) and DNA probe (blue). Central macrophage is indicated by an arrow and a multilobulated reticulocyte by an arrowhead. (Panels A and B are courtesy of Michel Prenant of France, and panel C is reprinted from Lee et al24 with permission from the American Society of Hematology.) Illustration by Paulette Dennis.
). (C) Confocal immunofluorescence image of an island reconstituted from freshly harvested mouse bone marrow cells stained with erythroid-specific marker (red), macrophage marker (green) and DNA probe (blue). Central macrophage is indicated by an arrow and a multilobulated reticulocyte by an arrowhead. (Panels A and B are courtesy of Michel Prenant of France, and panel C is reprinted from Lee et al24 with permission from the American Society of Hematology.) Illustration by Paulette Dennis.
The study of erythropoietic niches was extended in the 1970s by reconstruction of 3-dimensional scale models of bone marrow from a hypertransfused and a normal rat using 500 serial sections.3 In the normal rat bone marrow, all erythroblasts were in intimate contact with macrophages and a majority of the islands contained erythroblasts at various stages of differentiation. In contrast, in the hypertransfused rat, erythroblasts underwent developmentally synchronous proliferation and differentiation in distinct erythroblastic islands. Erythroblasts in individual clusters were composed of 2, 4, 8, or 16 cells at the same stage of morphologic maturation. Of note, in both normal and hypertransfused rats, erythropoiesis was not spatially restricted to the areas proximal to sinusoids but was found to occur over the entire marrow space. Strikingly, the number of islands decreased 8- to 10-fold after increasing the rats' hematocrits from 45% to 70%. These observations provided convincing evidence that suppression of erythropoiesis leads to a decrease in erythroblastic islands and that the level of erythropoietic activity is linked to island number.
The advent of long-term bone marrow cultures pioneered by Dexter and Allen in the 1980s produced further insights into erythroid niches.4 Long-term liquid cultures of mouse bone marrow, consisting of marrow stromal cells and extracellular matrix proteins under appropriate culture conditions for stimulating erythropoiesis, generated erythroblastic islands in vitro. Individual islands consisted of one or more synchronously maturing cohort of erythroid cells undergoing 4 or 5 divisions between proerythroblast and normoblast. Each island was centered on a macrophage, with large areas of closely opposed erythroblast and macrophage membranes. At the conclusion of terminal differentiation, expelled nuclei were phagocytosed by the central macrophage (Figure 2).
Proliferation and differentiation processes occurring within the erythroid niche. Early-stage erythroblasts are larger cells with centrally located nuclei; more differentiated erythroblasts are smaller cells containing nuclei located adjacent to plasma membranes. Expelled nuclei undergo phagocytosis by central macrophage. Young multilobulated reticulocytes are initially attached to the macrophage surface and later detach. Illustration by Paulette Dennis.
Proliferation and differentiation processes occurring within the erythroid niche. Early-stage erythroblasts are larger cells with centrally located nuclei; more differentiated erythroblasts are smaller cells containing nuclei located adjacent to plasma membranes. Expelled nuclei undergo phagocytosis by central macrophage. Young multilobulated reticulocytes are initially attached to the macrophage surface and later detach. Illustration by Paulette Dennis.
Despite this insightful and prophetic description of erythroid niches, these findings did not elicit further study until recently. During the interim, emphasis on the study of erythropoiesis was focused primarily on erythropoietin (Epo), identification of the Epo receptor and its signaling cascade; erythroid transcription factors; and the role of the locus control region in regulation of globin gene expression and regulation of hemoglobin switching. While findings from these investigations have had an enormous impact on our molecular understanding of terminal erythroid differentiation, it should be noted that many of these studies were not conducted in the context of erythroid niches, where all erythropoiesis occurs in vivo. Hence, the current challenge is to achieve a similar degree of molecular insight into positive and negative regulation of terminal erythroid differentiation in erythroid niches by adhesive interactions, central macrophage function, and various cytokines and chemokines. Coupling of such insights with our previous knowledge of erythropoiesis will be essential for understanding the tightly regulated production of red cells in both steady-state and stress erythropoiesis.
Island composition and localization
Although methodologies have evolved, studies over the past 50 years have provided consistent data regarding the cellular composition of erythroid islands and their localization within the marrow. In steady-state erythropoiesis, cells from proerythroblasts through multilobulated, young reticulocytes surround central macrophages.5-9 The number of erythroblasts per island ranges from 10 cells observed in tissue sections from rat femur9 to 5 to more than 30 erythroblasts in islands harvested from human marrow.7 A key component of erythroid islands is the central macrophage. To date, genomic and proteomic characterization of these cells is incomplete. Bone marrow contains a heterogeneous population of monocytes/macrophages at various differentiation stages with diverse phenotypes. Island macrophages, derived from monocyte precursors, are likely to be a subset of resident macrophages in hematopoietic tissue. Central macrophages harvested from human bone marrow islands express CD4, CD11a, CD11c, CD18, CD31, HLA-DR and FcRI, FcRII, FcRIII.7 In the murine system, F4/80 antigen and Forssman glycosphingolipid most reliably distinguish central macrophages from other hematopoietic stromal cells. F4/80 antigen, a surface glycoprotein, possesses homology to the G-protein–linked 7 transmembrane-spanning family of hormone receptors.10-12 Forssman glycosphingolipid discriminates island macrophages from marrow macrophages cultured with macrophage colony-stimulating factor (M-CSF) and from monocytes.13-15 Ideally, investigations of central macrophage function should be performed using macrophages isolated directly from islands.
Interestingly, in contrast to megakaryocytes, erythroid islands localize not only to regions adjacent to bone marrow sinusoids but also to regions throughout the marrow. One recent study using quantitative light and electron microscopy of rat bone marrow compared the differentiation stages of erythroblasts in 80 islands, 40 adjacent to sinusoids and 40 nonadjacent.16 Of note, nonadjacent islands contained significantly more proerythroblasts while islands neighboring sinusoids had more differentiated acidophilic erythroblasts. These data raise the intriguing possibility that islands may be motile structures migrating toward sinusoids as their erythroblasts become more differentiated. A speculative mechanism could entail crosstalk between central macrophages and maturing erythroblasts that stimulates secretion of macrophage proteases capable of remodeling extracellular matrix, thereby disrupting matrix attachments, releasing islands from their sites of origin, and enabling island migration toward sinusoids. An alternative possibility is that maturing erythroblasts move toward sinusoids by detaching and reattaching to stationary macrophages positioned at various distances from sinusoids. Such a mechanism could explain the appearance of erythroblasts in the circulation during periods of stress erythropoiesis, if a large increase in the number of erythroblasts during stress is greater than the number of reattachment sites on macrophages.
Adhesive interactions within islands
During definitive erythropoiesis, erythroblasts are known to express a diverse array of adhesion molecules that undergo dynamic variation during differentiation. These proteins mediate both erythroblast/erythroblast and erythroblast/macrophage interactions (Figure 3), as well as attachments to extracellular matrix components such as fibronectin and laminin. Historically, Emp (erythroblast macrophage protein) was the first molecule identified in both erythroblast and macrophage membranes that appears capable of forming macrophage/erythroblast attachments via homophilic binding.17 Culturing erythroblasts in the presence of anti-Emp or in the absence of macrophages results in a dramatic decrease in proliferation, maturation, and enucleation, associated with a 6-fold increase in apoptosis.18 During fetal liver macrophage differentiation in vitro, Emp appears to localize to different intracellular sites in immature macrophages while in mature cells it is largely present on the cell surface.19 In these experiments, mature macrophages bound erythroblasts while immature macrophages did not. Adding strength to the argument that Emp performs a critical role(s) in erythropoiesis is the finding of severe anemia and perinatal death in Emp-null fetuses.20
Erythroblast-macrophage adhesive interactions in the erythroid niche. Interactions illustrated include erythroblast α4β1 binding macrophage VCAM-1; erythroblast ICAM-4 binding macrophage α⋁ integrin; homophilic binding mediated by Emp between erythroblast and macrophage. Illustration by Paulette Dennis.
Erythroblast-macrophage adhesive interactions in the erythroid niche. Interactions illustrated include erythroblast α4β1 binding macrophage VCAM-1; erythroblast ICAM-4 binding macrophage α⋁ integrin; homophilic binding mediated by Emp between erythroblast and macrophage. Illustration by Paulette Dennis.
The second receptor/counterreceptor identified as mediating cell-cell interactions within erythroid islands were α4β1 integrin in erythroblasts and VCAM-1 in central macrophages.21 In support of this interaction, antibodies to either α4β1 or VCAM-1 have been shown to disrupt erythroblastic islands.21 Interestingly, this receptor/counterreceptor interaction also contributes to the integrity of erythroblastic islands formed by yolk sac–derived primitive erythroblasts and fetal liver macrophages. Primitive erythroblasts, which initially circulate as nucleated cells, enucleate later (between embryonic day [E] 14.5-16.5 of mouse gestation)22 after interaction with fetal liver macrophages.23 During this period of gestation, F4/80 positive liver macrophages are in close proximity to late stage primitive erythroblasts in vivo and erythroid islands composed of primitive erythroblasts can be harvested from fetal livers. In culture, enucleation does not occur unless late stage primitive erythroblasts are cocultured with fetal liver macrophages. Together this evidence convincingly shows that enucleation occurs within fetal liver erythroblastic islands. As in definitive erythropoiesis, α4 blocking antibody reduces the number of islands, as well as the number of erythroblasts per island.23 Moreover, VCAM-1 is the likely counterreceptor for α4 because E15.5 F4/80 positive liver cells express VCAM-1 on their surface.
More recently we discovered another set of adhesion molecules that contribute to island integrity; erythroid intercellular adhesion molecule-4 (ICAM-4) and macrophage αV integrin. A 70% decrease in islands is observed upon blocking ICAM-4/αV binding with αV synthetic peptides.24 Further, in ICAM-4 null mice, islands formed in vivo or reconstituted in vitro are strikingly decreased.24 Taken together, these observations present persuasive evidence that ICAM-4/αV attachment contributes to island integrity. The discovery of ICAM-4S, a novel secreted isoform of mouse ICAM-4 that is up-regulated late in terminal differentiation,25 signifies that secreted molecules may compete with membrane-bound ICAM-4 for macrophage αV counterreceptors, thus blocking ICAM-4/αV adhesion. A currently postulated function for ICAM-4S is to enable young reticulocytes to detach from central macrophages, thereby allowing them to enter the circulation.
A number of additional macrophage surface proteins have been identified as receptors for erythroblasts, although their counterreceptors remain uncharacterized. One such protein, originally named SER (receptor for sheep erythrocytes)26,27 but now termed sialoadhesin (CD69),28 binds erythroblast sialylated glycoproteins and localizes to sites of macrophage/erythroblast contact in erythroid islands.29 Another macrophage adhesion glycoprotein with an unidentified erythroid binding partner is CD163 (formerly called ED2 antigen30 ), which functions as a receptor for hemoglobin-haptoglobin complexes and is felt to be involved in the clearance of free hemoglobin.31 In vitro, CD163 enhances erythroid expansion but does not alter differentiation.31 It is highly likely that future studies will identify additional attachments between cells within erythroid islands.
Central questions that need to be addressed include: what is the function(s) of the various erythroblast/macrophage linkages; and are these interactions dynamic and relevant to specific developmental stages? Because association of integrins with the actin cytoskeleton regulates intracellular signaling (as reviewed in DeMali et al32 ), one can speculate that certain of these adhesive linkages may initiate signaling pathways coordinating gene expression and adhesion.
Role of central macrophages
Central macrophages not only anchor erythroblasts within island niches and provide interactions that affect erythroid proliferation and/or differentiation, but they also perform other functions. Studies in the 1960s33,34 and later document that at conclusion of terminal differentiation extruded erythroblast nuclei undergo phagocytosis by central macrophages. One intriguing question is how expelled nuclei remain localized at the macrophage surface after extrusion. Pertinent to this question is an analysis of partitioning of adhesion molecules between reticulocytes and expelled nuclei during enucleation. Recently we and others have learned that Emp20,35 and β1 integrin35 partition predominantly to extruded nuclei; this sorting pattern of adhesion molecules would effectively enable nuclei to maintain macrophage attachments before phagocytosis. A related study describes how nuclei expelled from fetal liver erythroblasts expose phosphatidylserine (PS) on their surface within a 10-minute period and are subsequently phagocytosed by fetal liver macrophages.36 This 10-minute timeframe is entirely consistent with earlier time-lapse observations of long-term Dexter cultures in which 10 minutes elapsed between nuclear expulsion and phagocytosis of the expelled nucleus.37 Movement of PS from the inner to the outer leaflet of the lipid bilayer is likely the result of observed decreased ATP inactivating the aminophospholipid translocase and increased Ca2+ levels activating the scramblase.36 Further, a mutant of milk-fat-globule EGF836 that binds surface PS38 and inhibits phagocytosis of apoptotic cells also blocks phagocytosis of extruded erythroblast nuclei36 ; strong evidence that the recognition signal for expelled nuclei is similar to apoptotic cells. A protective macrophage function intimately tied to phagocytosis of extruded nuclei has only recently been delineated; in normal mice DNase II in macrophages degrades the ingested nuclear DNA.39 In contrast, phagocytosed nuclei accumulate in F4/80 positive central macrophages of DNase II knockout mice but their DNA is not degraded. The undegraded nuclei appear to be toxic as there are significantly fewer F4/80 macrophages associated with erythroblasts, in conjunction with severe anemia.
Another important role originally proposed for central macrophages is transfer of iron directly to attached erythroid progenitors.2 A very recent report suggests that in macrophage/erythroblast coculture studies under transferrin-free conditions, ferritin is synthesized by macrophages, secreted via exocytosis, and subsequently taken up by erythroblasts. After endocytosis, iron, released from ferritin by acidification and proteolysis, is used by erythroblasts for heme synthesis.40 Although the attractive hypothesis of transfer of iron from central macrophages to erythroblasts has not yet been conclusively proven, with the exciting advances in our knowledge of iron metabolism and iron transporters answers should soon be forthcoming.
Abnormalities in macrophage differentiation can lead to perturbations in the function of the erythroid niche. One important factor regulating macrophage differentiation is retinoblastoma tumor suppressor (Rb) protein,41 a nuclear protein that functions in regulating the G1-to-S-phase transition of the cell cycle. A series of studies in the 1990s reported that Rb-null fetuses are anemic and die in utero.42-44 The finding that definitive fetal liver erythroblasts differentiate abnormally and fail to enucleate implies that Rb function is necessary for creating a normal erythroid niche. To date, the molecular and mechanistic basis for perturbed Rb null erythropoiesis has not been fully resolved. Two lines of evidence have been reported: (1) Rb null macrophages do not complete differentiation, because normally functioning Rb, which counteracts inhibition of Id2 on PU.1 (a transcription factor regulating macrophage differentiation), is absent41 ; (2) Rb-null macrophages function abnormally because of hypoxic stress present in the Rb-null fetal liver microenvironment.45 Moreover, there is strong evidence accumulating that an intrinsic erythroblast defect also contributes to anemia in Rb-null mice. In vitro, Rb-null erythroid differentiation is perturbed42 with cells not exiting the cell cycle or enucleating normally, irrespective of macrophage function.45,46 Further, conditional gene inactivation reveals that Rb couples mitochondrial biogenesis with cell-cycle exit.47 Future studies will be essential to fully resolve the relative contributions of macrophage and erythroblast dysfunction to the Rb-null phenotype.
A second protein affecting central macrophage function is cytoskeletal-associated protein palladin.48 This protein, localizing in focal adhesions of stress fibers along with alpha-actinin, regulates actin cytoskeleton dynamics and cell attachment to extracellular matrix. The targeted deletion of palladin is embryonic lethal by E15.5 with an erythroid phenotype including severe anemia developing at E13.5; a marked decrease in definitive erythrocytes; and increased erythroblast apoptosis accompanied by partially blocked terminal differentiation. Moreover, fetal liver erythroblastic island structures are disorganized in vivo and palladin-null fetal liver cells are incapable of forming islands in vitro. Studies to identify the malfunctioning island component reveal that palladin-null macrophages do not form islands with either wild-type (WT) or mutant erythroblasts, while WT macrophages form islands with both WT or palladin-null erythroblasts. These data convincingly show that the defect is intrinsic to macrophages and that palladin deficiency interferes with fetal liver erythroid island formation. Yet to be defined is the mechanism by which palladin deficiency perturbs macrophage cytoskeletal function and the downstream molecular effects of that perturbation.
Positive regulatory feedback within islands
Erythropoiesis is driven by the balance between positive and negative feedback mechanisms operating within the island niche involving both cell-cell interactions and soluble factors. The concentration of Epo is a predominant regulator of erythroid progenitor numbers by preventing apoptosis of cells in the colony-forming unit erythroid (CFU-E)/proerythroblast stages of differentiation.49 However, cell-cell interactions within islands also play crucial roles. Indeed, erythroblasts interacting with one another function to regulate erythroid lineage output. In a study of human erythropoiesis, Fas/Fas ligand binding appears to contribute to regulation of apoptosis of immature erythroblasts.50 Although Fas is expressed on human erythroblasts at all stages of terminal differentiation, only in immature erythroblasts does Fas crosslinking transduce a death signal. Fas ligand is not expressed until late in differentiation and orthochromatic erythroblasts demonstrate a Fas-based cytotoxicity against immature erythroblasts. However, in vitro, increased Epo consistent with levels measured in anemia, provides protection to early erythroblasts from Fas-induced cytotoxicity by late erythroblasts. By enabling more immature erythroblasts to terminally differentiate, this mechanism effectively up-regulates erythropoiesis in the presence of elevated Epo. During mouse fetal erythropoiesis, Fas/Fas ligand binding also appears to regulate the number of erythroblasts reaching maturity; however, in fetal mice, Fas-mediated apoptosis results from interactions between immature erythroblasts.51 Further investigations will be needed to determine whether the observed differences in the stage of differentiation of erythroblasts inducing apoptosis in humans compared with fetal mice represents species variation and/or a difference between adult and fetal erythropoiesis. Importantly, however, both studies support a role for Fas/Fas ligand binding as an active regulator of apoptosis within erythroblastic islands. Interestingly, increased Fas expression has been reported in CD34+ cells from individuals with aplastic anemia, indicating that Fas-based cytotoxicity may contribute to bone marrow suppression in this severe disorder (as reviewed in Niho et al52 ).
Erythroid progenitor numbers can be regulated not only by protection from apoptosis but also by cell-cell interactions that increase proliferation. Direct contact with macrophages enhances erythroblast proliferation.53 Erythroid islands form when CFU-E/proerythroblasts are cultured with macrophages. Erythroblasts in direct contact with macrophages proliferate 3-fold more than erythroid progenitors cultured alone. BrdU pulse-chase experiments exploring the mechanism for enhanced proliferation reveal a decreased transit time in the G0/G1 phase of the cell cycle. The observed 3-fold enhancement of proliferation signifies between 1 and 2 additional cell divisions, which would substantially increase the final number of reticulocytes generated. Importantly, the same degree of enhanced proliferation is observed in the presence of a physiologic range of Epo concentrations, clearly demonstrating that increased proliferation is regulated by a molecular mechanism other than the antiapoptotic effect of Epo. These findings underscore the role of macrophage/erythroblast attachment in regulation of red cell production. Furthermore, they support the concept that perturbations in macrophage function can result in anemias that are less responsive to treatment with Epo, as is observed in anemias associated with malignancy and chronic inflammation.
An open question is which erythroblast/macrophage attachment(s) governs the change in cell-cycle dynamics. Is it one of the adhesive interactions described above or does it involve other macrophage surface proteins? Two potential candidate interactions have been identified as enhancing erythroblast proliferation at the CFU-E stage. Macrophage membrane protein Ephrin-2 (HTK ligand) interacting with its erythroid receptor EphB4 (HTK)54,55 and the c-kit ligand transmembrane protein binding c-kit on erythroid progenitors.56 Although the precise identity of the receptor(s)/counterreceptor(s) regulating erythroid cell-cycle dynamics have yet to be definitely established, accumulated evidence is now conclusive that macrophages are central to this process. Further underscoring their importance is the finding that the appropriate erythroid response to bleeding is prevented in mice chemically depleted of splenic macrophages.57
Erythroblast-erythroblast attachments also regulate cell proliferation. It has long been recognized that the level of transcription factor GATA-1 is crucial to completion of normal differentiation.58 Interestingly, while proerythroblasts undergo apoptosis in the absence of GATA-1,59-63 its overexpression inhibits terminal differentiation.64,65 A recent study documents the importance of homotypic signaling between erythroblasts within island niches as a mechanism for regulating GATA-1 activity. It has been observed in a mouse model in which half of the erythroblasts lack GATA-1 and half overexpress GATA-1 that the defect(s) in GATA-1 overexpressing cells can be corrected by a signal, termed REDS (red cell differentiation signal), produced by normal late erythroblasts.58 REDS is not a soluble factor and the mechanism of its activity appears to require erythroblast/erythroblast interaction. These observations strongly suggest that GATA-1 activity and hence, gene expression, are regulated by intercellular signaling between erythroblasts.
Soluble factors secreted by macrophages add another layer of positive regulation within erythroblastic islands. Macrophage cytokines, including burst-promoting activity and insulin-like growth factor-1, induce growth of both burst forming unit-erythroid (BFU-E) and CFU-E.66-68 In addition, Epo mRNA was detected in macrophages in one report 20 years ago,69 although there has been no follow-up confirmation.
Soluble factors secreted by erythroblasts also have relevant functions within the erythroid niche. A very recent discovery is that Gas6, a secreted protein that enhances proliferation and survival in nonerythroid cells, is released by erythroblasts in response to Epo.70 Secreted Gas6 binds its erythroblast receptor, and by activating PI3K and its effector Akt, enhances Epo receptor signaling. Further, binding of Gas6 to its receptor decreases macrophage secretion of erythroid inhibitory factors within the erythroid niche.70 Hence, Gas6 deserves to be explored as a potential therapy for patients with anemias that do not adequately respond to Epo. Differentiating erythroblasts also secrete angiogenic factors, vascular endothelial growth factor A (VEGF-A) and placenta growth factor (PIGF).71 Media from cultured erythroblasts induces monocyte migration and increased endothelial cell permeability, that are inhibited by antibodies to VEGF-A and PIGF. Erythroblasts have no receptors for VEGF-A or PIGF, hence these secreted angiogenic factors may serve as paracrine effectors, mediating crosstalk between receptor-expressing macrophages and developing erythroblasts that regulates island structure and/or localization. In addition, by affecting endothelial cell junctional integrity these secreted proteins may facilitate reticulocyte movement into bone marrow sinusoids.
Negative regulatory feedback within islands
Elevated levels of circulating cytokines, chemokines, and interleukins, including interleukin 6 (IL6), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and interferon-γ (INF-γ), characterize chronic inflammation and malignancy. In these disorders bone marrow constituents are bathed in plasma containing increased amounts of these factors. It is also likely that local concentrations may be even higher within erythroid niches because of direct secretion by central macrophages (Figure 4). In patients with chronic inflammation, cytokines in bone marrow have been associated with inhibition of erythropoiesis.72 This suppression is mechanistically complex and multifactorial, not only because it is induced by multiple cytokines, chemokines and interleukins but also because each of these proteins by itself may have more than one effect upon erythroid development. TNF-α inhibits erythropoiesis either by caspase-mediated cleavage of the major erythroid transcription factor GATA-1 resulting in apoptosis50 and/or by retarding proliferation73 ; the precise mechanism(s) remains somewhat controversial. In nonerythroid tissues, TNF-α stimulates macrophages to secrete metalloproteinases, a number of which have the capacity to remodel extracellular matrix. Such an effect occurring within erythroid niches would perturb matrix-erythroblast adhesive attachments. In patients with MDS, bone marrow macrophages secrete high levels of TNF-α.74 Hence in MDS and chronic inflammatory states, normal macrophage-induced erythroblast proliferation may be inhibited by TNF-α. TGF-β also perturbs erythropoiesis in various ways. It inhibits erythroblast proliferation by reducing the number of progenitors in cycle via a mechanism other than apoptosis75 and accelerates differentiation of noncycling erythroid progenitors.75 TGF-β triggers activation of Rho and Rac GTPases thereby reorganizing actin cytoskeleton in a variety of nonerythroid cells (for example, see Maddala et al76 ); when this occurs in erythroblasts it could have pronounced effects on various cytoskeletal functions including membrane stability. Elevated levels of INF-γ induce both macrophages and erythroblasts to secrete soluble TRAIL (TNF-related apoptosis inducing ligand), which inhibits erythroblast differentiation77 mediated by activation of the intracellular ERK/MAPK pathway.78 Finally, IL6 up-regulates hepcidin expression, which inhibits iron export from macrophages by binding to the iron exporter ferroportin and inducing its internalization and degradation, thereby blocking availability of iron for erythropoiesis (as reviewed in Nemeth et al79 ).
Schematic representation of negative regulatory factors in erythroid niche. Erythropoiesis is affected by (1) increased circulating levels of cytokines, chemokines and interleukins; (2) secretion of these factors by the central macrophage; and (3) perturbations in the extracellular matrix. Illustration by Paulette Dennis.
Schematic representation of negative regulatory factors in erythroid niche. Erythropoiesis is affected by (1) increased circulating levels of cytokines, chemokines and interleukins; (2) secretion of these factors by the central macrophage; and (3) perturbations in the extracellular matrix. Illustration by Paulette Dennis.
Another negative feedback loop regulating erythropoiesis within islands involves a lesser known soluble factor secreted by bone marrow macrophages termed RCAS1 (receptor binding cancer antigen expressed in SiSo cells).80 The binding of RASC1 to its receptor expressed on immature erythroblasts activates proapoptotic caspases 8 and 3. Hence, RASC1 represents another regulator of apoptosis of erythroid progenitors. We look forward to future investigations delineating the relative role of RASC1 in normal and/or disordered erythropoiesis.
In sum, there are myriad means by which elevated levels of circulating cytokines, chemokines, and interleukins can disorder erythropoiesis and there is a pressing need to dissect out the various molecular mechanisms and their potential interplay to design novel therapies to stimulate effective erythropoiesis.
Extracellular matrix in erythroid niches
Currently 2 extracellular matrix proteins, fibronectin and laminin, are strong candidates for regulating processes in terminally differentiating erythroblasts and reticulocytes. Fibronectin influences growth, differentiation, adhesion, and migration of multiple cell types, including hematopoietic cells. Erythroblasts express 2 integrins that bind fibronectin, α4β1 and α5β1 (as reviewed in Hanspal81 ). However α5β1 expression is down-regulated during terminal differentiation and late-stage erythroblasts express almost exclusively α4β1 on their surface.82 α4β1 mediates adhesion to several sites on the fibronectin glycoprotein. The alternatively spliced IIICS region contains 2 α4β1 recognition sequences, LDV within the CS1region and REDV within the CS5 region.83-85 A lower-affinity α4β1 binding site is located in the HepII domain.86 In the later stages of erythroid differentiation a progressive loss of adherence to fibronectin occurs, with reticulocytes being nonadherent.87 When murine erythroleukemia cells are cultured on fibronectin rather than plastic they differentiate into reticulocytes more extensively; furthermore, enucleated cells can be observed detaching from the fibronectin matrix.88 A recent exciting finding is that proliferation appears to be regulated by an early Epo-dependent phase followed by an α4β1 integrin/fibronectin-dependent phase.89 Both Epo and fibronectin promote proliferation by protecting cells from apoptosis, partly through antiapoptotic bcl-xL. Together, these studies convince us that erythroblast attachment to fibronectin plays a functional role in erythropoiesis, particularly erythroid expansion.
Late in terminal differentiation Lutheran (Lu) glycoproteins that bind extracellular matrix laminin appear on the erythroblast surface.90-92 Lu glycoproteins are 85- and 78-kDa isoforms that are members of the immunoglobulin superfamily (IgSF).93-95 The extracellular regions of both isoforms are identical, containing 5 IgSF domains. The cytoplasmic tail of the 85-kDa isoform has a SH3 binding domain and several potential phosphorylation sites, suggesting a signal transduction function, while the 78-kDa isoform (also termed B-CAM) has a truncated cytoplasmic tail. We determined that Lu is a specific, high-affinity receptor for laminins containing the α5 polypeptide chain, specifically laminin10/11 (also termed laminin 511/521),96 and that the laminin-binding site is located at the flexible junction of Ig domains 2 and 3.97 Various laminins localize to different regions of extracellular matrix. Interestingly, laminins 10/11 are present in the subendothelial basement membrane of bone marrow sinusoids.98 Based on the timing of Lu expression late in human erythroid differentiation and the localization of its laminin binding partner to basement membrane of bone marrow sinusoids, it is reasonable to speculate that this adhesive interaction may function to localize reticulocytes to sinusoids, as the initial step of their entrance into the peripheral circulation.
Conclusions
The well-documented ability of erythroid precursors to complete terminal differentiation in vitro in the presence of Epo but without either macrophage or extracellular matrix contacts demonstrates that an autonomous differentiation program exists in committed progenitors. However, under these conditions the extent of proliferation and differentiation, as well as efficiency of enucleation, in no way approaches what has been documented in vivo. Thus an autonomous program working in concert with adhesive interactions, cellular cross talk, and regulatory feedback pathways within erythroid niches is necessary to generate 2 × 106 reticulocytes per second in steady state, with an ability to increase production by 15- to 20-fold under stress conditions.
Future directions
Cell-cell and cell-matrix adhesive interactions are hallmarks of erythroid niches and one of the future challenges is to determine the functional sequeli of these various attachments. The intriguing questions that need to be answered include the following: What are the dynamic profiles of various adhesive attachments during terminal differentiation? Which of these adhesive interactions initiate signaling pathways to regulate gene expression and/or reorganize cytoskeleton? How does the central macrophage enhance erythroid progenitor proliferation and traffic iron to developing erythroblasts? Does the central macrophage remodel extracellular matrix in its vicinity enabling the movement of islands toward sinusoids to facilitate the release of reticulocytes into circulation? Additionally, we need to develop a detailed mechanistic understanding of the various positive and negative regulatory factors operating upon the erythroid niche that modulate normal, as well as ineffective and stress erythropoiesis. We anticipate these avenues of research will lead to the application of novel therapies, such as anti-TNFα and Gas6, that could be used alone or in combination with Epo to effectively treat disorders including anemia of chronic inflammation and malignancy, MDS, thalassemias and malarial anemia. We hope all these questions will be answered in time to celebrate the 75th anniversary of the original description of erythroblastic islands.
Acknowledgments
The authors thank Gloria Lee for her contribution to preparation of the figures for this manuscript. This review was supported in part by National Institutes of Health grants DK56267, HL7956, and DK32094 and by the Director, Office of Health and Environment Research Division, US Department of Energy, under contract DE-AC03-76SF00098.
National Institutes of Health
Authorship
Contribution: J.A.C. and N.M. cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Joel Anne Chasis, Lawrence Berkeley National Laboratory, Building 74, One Cyclotron Road, Berkeley, CA 94720; e-mail: jachasis@lbl.gov.