Abstract
CD4+CD25highFoxP3+ regulatory T (Treg) cells limit antigen-specific immune responses and are a cause of suppressed anticancer immunity. In preclinical and clinical studies, we assessed the immune consequences of FoxP3+ Treg-cell depletion in patients with advanced malignancies. We demonstrated that a CD25high targeting immunotoxin (denileukin diftitox) depleted FoxP3+ Treg cells, decreased Treg-cell function, and enhanced antigen-specific T-cell responses in vitro. We then attempted to enhance antitumor immune responses in patients with carcinoembryonic antigen (CEA)–expressing malignancies by Treg-cell depletion. In a pilot study (n = 15), denileukin diftitox, given as a single dose or repeated dosing, was followed by immunizations with dendritic cells modified with the fowlpox vector rF-CEA(6D)-TRICOM. By flow cytometric analysis, we report the first direct evidence that circulating CD4+CD25highFoxP3+ Treg cells are depleted after multiple doses of denileukin diftitox. Earlier induction of, and overall greater exposure to, the T-cell response to CEA was observed in the multiple-dose group, but not the single-dose group. These results indicate the potential for combining Treg-cell depletion with anticancer vaccines to enhance tumor antigen-specific immune responses and the need to explore dose and schedule of Treg depletion strategies in optimiz-ing vaccine efforts. This trial was registered at www.clinicaltrials.gov as no. NCT00128622.
Introduction
Vaccines to treat cancer or prevent its recurrence have been of considerable interest,1,2 but among the challenges to their efficacy have been an immune-suppressive effect of the tumor microenvironment and modulation of T-cell expansion by inhibitory cells such as regulatory T (Treg) cells. Treg cells, defined by their expression of CD4, persistently high expression of the interleukin-2 (IL-2) receptor component CD25, and intracellular expression of the transcription factor FoxP3,3 prevent uncontrolled proliferation of antigen-specific T cells.4-7 Increasing evidence implicates a contribution of Treg cells to the impaired host immune response against cancer.8,9 Elevated Treg-cell levels in the peripheral blood, regional lymph nodes, and tumor microenvironment of patients with cancer are associated with reduced survival.10-13 Depletion of Treg cells in animal models leads to enhanced antitumor immune responses.14-17 A recent human study reported that Treg-cell depletion before immunization enhanced tumor antigen-specific T-cell responses.18
Potential strategies for depleting Treg cells, such as anti-CD25 antibodies and cyclophosphamide, are limited by the persistence of the antibodies that would also deplete or inhibit activated T cells that express CD25 and the nonspecific toxicity of chemotherapeutics. We therefore studied denileukin diftitox (ONTAK; Eisai, Woodcliff Lake, NJ), a fusion between the active domain of diphtheria toxin and IL-2. Denileukin diftitox binds to cells expressing high levels of CD25 whereupon it is internalized, leading to blockade of protein synthesis and cell death.19 Denileukin diftitox has direct antitumor activity against CD25-expressing T-cell malignancies.20 Cells expressing the high-affinity IL-2 receptor are most susceptible to the effects of denileukin diftitox, but the short half-life of denileukin diftitox (70-80 minutes) should limit its impact on subsequently activated effector T cells. In murine models and in humans with melanoma and renal cell carcinoma, denileukin diftitox reduced CD4+CD25high levels.18,21-23 With the availability of techniques to identify FoxP3 by permeabilization and intracellular staining, we can now directly assess the circulating levels of the more precisely defined CD4+CD25highFoxP3+ Treg cells over time.
To address the hypothesis that Treg-cell depletion would enhance immune responses against a cancer vaccine, we performed a preclinical study that demonstrated depletion of Treg cells before application of antigen in vitro–enhanced antigen-specific T-cell responses. Subsequently, patients with advanced carcinoembryonic antigen (CEA)–expressing malignancies were administered denileukin diftitox before immunization with a vaccine consisting of dendritic cells (DCs) modified with the viral vector rF-CEA(6D)-TRICOM vaccine. We observed depletion of CD4+CD25highFoxP3+ Treg cells and enhanced CEA-specific T-cell immunity.
Methods
Blood collection and cell preparation
Blood and leukapheresis products were obtained after signed informed consent from patients with cancer and healthy donors according to institutional review board (IRB)–approved protocols. Peripheral blood mononuclear cells (PBMCs) were separated over a Ficoll gradient. Relevant Duke University IRB or Ethics Committee approval was obtained for each trial.
Treg-cell depletion in vitro with denileukin diftitox detected using flow cytometry
PBMCs were cultured for 18 to 20 hours (day 1) in complete media (RPMI 1640, 10% huAB serum, 25 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM glutamine) with denileukin diftitox (0-8 nM), washed, and replated in complete media with 10 U/mL IL-2 for 3 days to allow recovery. On day 4, Treg cells were identified using an anti–human FoxP3 staining set (eBioscience, San Diego, CA). Briefly, cell-surface staining with anti-CD25–FITC, anti-CD3–PerCP, and anti-CD4–APC (BD Biosciences, San Jose, CA) was followed by fixation and permeabilization before intracellular staining with anti-Foxp3–PE. Events collected using a FACS Calibur (BD Biosciences) were analyzed using CellQuest (BD Biosciences). CD4+CD25+FoxP3+ T cells were identified by gating on the CD4+CD25high cells expressing more than 90% FoxP3+.
Functional analysis of Treg-cell depletion using proliferation assay
PBMCs treated with various concentrations of denileukin diftitox were analyzed for their proliferative capacity (on day 4) in response to stimulation with anti-CD3 (OKT3). PBMCs (105 cells/well in a total volume of 200 μL) were stimulated with media alone or 0.5 μg/mL soluble OKT3 for 96 hours. Proliferation was determined by [3H]thymidine (0.037 MBq/well [1 μCi/well]) incorporation using a Wallac scintillation counter (Gaithersburg, MD) and was reported as mean counts per minute plus or minus standard deviation.
In vitro analysis of antigen-specific T-cell expansion after Treg-cell depletion
PBMCs from healthy HLA-A*0201+ volunteers, treated as described in “Functional analysis of Treg depletion using proliferation assay” with 4 nM denileukin diftitox and recovered in 10 U/mL IL-2 for 3 days, were stimulated with 1 μg/mL CMV pp65 (495-503) peptide or 1 μg/mL MART-1 (27-35) peptide at 2 × 106 cells/well in 24-well plates. IL-2 (300 U/mL) was added every 3 days. For CMVpp65 expansions, stimulated cells were analyzed by peptide–MHC tetramer binding at days 7 and 10. For MART-1 expansion, responding cells were restimulated (at an 8:1 stimulator:responder [S:R] ratio) on day 8 using irradiated, autologous PBMCs pretreated with 4 nM denileukin diftitox and pulsed with 10 μg/mL MART-1 (27-35) peptide. On day 8 of the reculture, MART-1 expanded cells were analyzed by peptide–MHC tetramer (Beckman Coulter, San Diego, CA) staining. Cells were stained with αCD3-FITC, αCD8-PerCP, αCD4, αCD14, and αCD19-APC, and tetramer-PE. Tetramer-positive cells were identified by gating on the population of cells that were forward scatterlo side scatterlo CD4−CD14−CD19−CD3+.
Phase 1 clinical trial of denileukin diftitox plus DC-rF-CEA(6D)-TRICOM
Participants were recruited from the medical and surgical oncology clinics of Duke University Medical Center and provided signed informed consent approved by the Duke University Medical Center IRB in accordance with the Declaration of Helsinki before enrollment. The study was performed under a US Food and Drug Administration (FDA)–approved Investigational New Drug (IND) exemption. Participants were required to have a metastatic cancer expressing CEA defined by immunohistochemical analysis or elevated CEA in peripheral blood and adequate hematologic (white blood cell [WBC] > 4.0 × 103 cells/μL), renal (creatinine < 2.0 mg/dL), and hepatic (bilirubin < 1.5 mg/dL) function. They must have received and had progressive disease on prior therapy. They were excluded if they had had chemotherapy, radiation therapy, or immunotherapy within the previous 4 weeks, a history of autoimmune disease (including inflammatory bowel disease, presence of an active acute or chronic infection, HIV, or viral hepatitis), or if they had used immunosuppressives in the preceding 4 weeks.
Generation of vaccines
Participants underwent a 4-hour peripheral blood leukapheresis, and the product was separated by density gradient centrifugation over Ficoll in a cell separator (Cobe BCT, Lakewood, CO) to obtain PBMCs. The DC vaccine was generated using patient DCs and rF-CEA(6D)-TRICOM (manufactured by Therion Biologics, Cambridge, MA; supplied by Cancer Treatment Evaluation Program, National Cancer Institute, Bethesda, MD) as previously reported.2
For vaccines that were to be administered fresh, the DC preparations were washed and resuspended at 5 × 106 cells in 0.5 mL saline. They were then mixed with the rF-CEA(6D)-TRICOM (25 × 106 particles) in a total volume of 1 mL saline for at least 30 minutes before administration. All cellular products were required to pass lot release criteria consisting of expression of HLA-DR and CD86 in at least 50% of the (large, dendritic) cells, viability greater than 70%, and no evidence of bacterial or fungal contamination. For vaccines administered after cryopreservation of the DCs, one vial of DC product was thawed and the required number of DC (5 × 106 cells) was mixed with the rF-CEA(6D)-TRICOM (25 × 106 particles) in a total volume of 1 mL saline for at least 30 minutes before administration. We found no significant differences in the mean expression of CEA, CD80, CD54, or CD58 between cohorts or between DCs that were fresh and those previously cryopreserved (data not shown).
Protocol schema and patient treatment
This phase 1 study enrolled patients into 2 sequential cohorts. In the first cohort (cohort 1), patients received one dose of denileukin diftitox 18 μg/kg intravenously 4 days before initiating DC injections. DCs were given every 3 weeks for 4 immunizations. Immunizations were given as a combination of intradermal (0.1 mL) and subcutaneous (0.9 mL) into the same limb (predominantly the right thigh), and injection sites were separated by 10 cm from each other.
The study protocol permitted escalation to the second cohort (cohort 2) if no more than 1 of the first 6 patients experienced a dose-limiting toxicity related to the vaccine. In this cohort, based on data suggesting better Treg-cell depletion with a lower dose of denileukin diftitox,24 patients received denileukin diftitox 9 μg/kg intravenously 4 days before every DC injection.
To compare the clinical and immunologic data from patients who received denileukin diftitox with data from patients who received the same vaccine, but no denileukin diftitox, we collected further clinical and immunologic data from patients who had participated in our previous clinical trial of DCs modified with rF-CEA(6D)-TRICOM.2 We refer to this group as cohort 0.
Analysis of clinical and immunologic activity
Clinical activity was assessed by applying the Response Evaluation Criteria in Solid Tumors (RECIST) criteria to computed tomography (CT) or magnetic resonance imaging (MRI) scans obtained before and after all immunizations. For immunologic analyses, peripheral blood was drawn before each immunization and after the final immunization. Fresh PBMCs were analyzed for antigen-specific reactivity. Blood was also drawn before and 4 days after each denileukin diftitox infusion for Treg-cell analysis (performed as described). For cohort 0, PBMCs from cryopreserved samples before and after immunizations were analyzed.
Enumeration of Treg cells
Whole blood from patients in cohort 2 was stained using anti-CD25, anti-CD4, anti-CD3, and anti-CD45 using BD Bioscience TruCount tubes. Using the percentage of CD4+CD25+ that were also FoxP3+ based on our intracellular staining described here, we determined the number of CD4+CD25+FoxP3+ cells per microliter of blood.
Analysis of CEA-specific T-cell responses from peripheral blood using IFNγ ELISPOT assay
Multiscreen-HA 96-well plates (Millipore, Bedford, MA) were coated overnight with 10 μg/mL mouse anti–human IFNγ Mab (diaPharma Group, West Chester, OH) and plated at 100 000 or 250 000 PBMCs/well in the presence of rF-CEA(6D)-TRICOM (multiplicity of infection [moi], 10), rF-wild type (moi, 10), CAP-1 peptide (1 μg/mL), CEA peptide mix (overlapping peptides spanning entire CEA molecule; 1360 μg/mL), CMVpp65 (495-503; 1 μg/mL), CMV peptide mix and HIV peptide mix (1.75 μg/mL/peptide; Pharmingen, San Diego, CA), PMA 2 μg/mL (Sigma-Aldrich, St Louis, MO), SEB 2 μg/mL (Sigma-Aldrich), or media. After a 18- to 20-hour incubation, 100 μL of mouse anti–human IFNγ biotinylated mAb (diaPharma Group) was added to each well for 2 hours followed by Vectastain ABC Peroxidase (Vector Labs, Burlingame, CA) at 100 μL/well for 1 hour. Color was developed using 100 μL/well AEC (Sigma-Aldrich).
Membranes were read using the KS enzyme-linked immunospot (ELISPOT) Automated Reader System with the KS ELISPOT 4.2 or 4.9 Software (Carl Zeiss, Thornwood, NY) to determine the number of spots per well. The mean number of spots from the 6 replicate wells was reported as the response to each antigen.
Analysis of CD4+ and CD8+ CEA-specific T-cell responses using intracellular cytokine staining
PBMCs (2-4 × 106/mL) were stimulated with the antigens (same concentrations as described for ELISPOT assay) for 6 hours (rF-CEA(6D)-TRICOM [moi, 1] and rF-wild type [moi, 1] incubated for 12 hours) in the presence of 10 μg/mL brefeldin A (Sigma-Aldrich) and 1 μg anti-CD28 (BD Biosciences) per 106 PBMCs. Stimulated cells were fixed with 1% paraformaldehyde and cryopreserved. Once all time points for a patient were collected, cells were permeabilized (FACS Permeabilizing Solution; BD Biosciences) and stained with αIFNγ-FITC, αCD69-PE, αCD8-PerCP, and αCD4-APC. The percentage of IFNγ/CD69 double-positive CD8+ T cells was determined within forward scatterlo side scatterlo CD8+ or CD4+ cells.
Analysis of anti-CEA antibodies by ELISA
Patient serum was collected before and after immunization. Anti-CEA and anti–fowlpox antibody (IgG) were quantified by enzyme-linked immunosorbent assay (ELISA). The 96-well plates were coated with CEA protein (100 ng/well), fowlpox–wild type (107 pfu/well), or KLH (100 ng/well; Sigma-Aldrich). Plates were incubated for 4 hours with 100 μL of serum serially diluted 1:10 to 1:31 250, then incubated with 100 μL alkaline phosphatase–conjugated goat anti–human IgG (working dilution: ×10 000; MP Biomedicals, Irvine, CA) for 3 hours, and developed with PNPP (p-nitrophenyl phosphate; Sigma-Aldrich). Reaction was stopped with 3 N NaOH and A405 nm was measured using the ELISA Plate Reader (Bio-Rad, Hercules, CA). Titers were defined as the highest dilution with A405 nm above background level (0.2 for CEA and KLH, 0.3 for fowlpox).
Statistical methods
The percentage change in CD4+CD25+FoxP3+ Treg cells from baseline (ie, before receiving denileukin diftitox [referred to as LPA]) for each cohort is determined by dividing the difference between each patient value for each time point and baseline by the baseline value (×100%).
Immune response data for ELISPOT and cytokine flow cytometric assays were captured for each patient at 0, 3, 6, 9, and 10 weeks. Area under the curve (AUC) of the immune response was estimated for each patient using the linear and log trapezoidal rules as follows. The linear trapezoidal rule was used to estimate the area of a segment when the curve was increasing; the log trapezoidal rule was used to estimate the area of a segment when the curve was decreasing. A linear trapezoidal segment is estimated as 1/2 (ti + 1 − ti) (mi + 1 + mi), where t denotes time point, m denotes the immune response measurement, and i is in (1, 2, … n − 1). Similarly, a log trapezoidal segment is estimated as (t i + 1 − ti)(mi + 1 − mi) / log(mi + 1 / mi). The total AUC for each patient is the sum of all segments. The Wilcoxon rank-sum test was used to compare the median AUC between cohorts. The t test was used to compare mean times to peak immune response between cohorts.
A positive immune response by ELISPOT assay was defined as described at the 2002 Society of Biologic Therapy Workshop on “Immunologic Monitoring of Cancer Vaccine Therapy”25 : a T-cell response is considered positive if the mean number of spots in 6 wells with antigen exceeds the mean number of spots in 6 control wells by 10 and the difference between the mean of the 6 wells containing antigen and the 6 control wells is statistically significant at a P value of .05 or less using the Student t test.25
Results
Denileukin diftitox depletes CD4+CD25highFoxP3+ Treg cells, leading to enhanced T-cell proliferation
PBMCs from healthy donors were treated with various concentrations of denileukin diftitox for 18 to 20 hours and stained for CD4, CD25, and FoxP3 expression (day 1). These cells were then washed and recultured in low-dose IL-2 containing media for an additional 3 days and again stained for CD4, CD25, and FoxP3 (day 4). The percentage of CD4+CD25highFoxP3+ Treg cells decreased (approximately 75%) when treated with increasing concentrations of denileukin diftitox (Figure 1A). This effect was observed on day 1 and was still apparent after the rest period (day 4), although a greater percentage of CD4+CD25highFoxP3+ Treg cells was observed at day 4. This suggests that Treg cells may experience a rebound effect due to the influence of the IL-2 during the rest period.
CD4+CD25highFoxP3+ Treg-cell levels and proliferation of PBMCs after in vitro treatment with denileukin diftitox. PBMCs from a healthy donor were treated with up to 8 nM denileukin diftitox or media alone (0 nM) for 18 to 20 hours and then cultured in media containing 10 U/mL IL-2 for 3 days. (A) Cells were stained for CD4, CD25, and Foxp3 expression on days 1 (after 18 hours of denileukin diftitox) and 4. The percentage of CD4+CD25highFoxp3+ cells are represented for each concentration. The percentage of CD4+CD25highFoxp3+ cells from freshly isolated PBMCs before the addition of denileukin diftitox is represented by a dashed line (1.82%). Data are representative of 3 repeated experiments. (B) PBMCs similarly depleted of Treg cells with denileukin diftitox and then rested in IL-2 for 3 days were stimulated in a proliferation assay with 0.5 μg/mL soluble OKT3 or media alone (unstimulated) for 4 days. Proliferation was determined by [3H]thymidine incorporation during a final 18 hours of culture, and the data are represented as mean cpm plus or minus SD. *P < .01 compared with 0 nM. Data are representative of 3 repeated experiments.
CD4+CD25highFoxP3+ Treg-cell levels and proliferation of PBMCs after in vitro treatment with denileukin diftitox. PBMCs from a healthy donor were treated with up to 8 nM denileukin diftitox or media alone (0 nM) for 18 to 20 hours and then cultured in media containing 10 U/mL IL-2 for 3 days. (A) Cells were stained for CD4, CD25, and Foxp3 expression on days 1 (after 18 hours of denileukin diftitox) and 4. The percentage of CD4+CD25highFoxp3+ cells are represented for each concentration. The percentage of CD4+CD25highFoxp3+ cells from freshly isolated PBMCs before the addition of denileukin diftitox is represented by a dashed line (1.82%). Data are representative of 3 repeated experiments. (B) PBMCs similarly depleted of Treg cells with denileukin diftitox and then rested in IL-2 for 3 days were stimulated in a proliferation assay with 0.5 μg/mL soluble OKT3 or media alone (unstimulated) for 4 days. Proliferation was determined by [3H]thymidine incorporation during a final 18 hours of culture, and the data are represented as mean cpm plus or minus SD. *P < .01 compared with 0 nM. Data are representative of 3 repeated experiments.
PBMCs depleted of CD4+CD25highFoxP3+ cells by denileukin diftitox pretreatment exhibited greater proliferation in response to anti-CD3 stimulation (OKT3) in a dose-dependent manner (Figure 1B). Because the peak proliferative effect plateaued at approximately 4 nM denileukin diftitox, the concentration achieved in the peripheral blood of patients treated with an 18 μg/kg dose, subsequent experiments used this concentration. Importantly, these results required the 3-day rest period and administration of the IL-2 to maintain viability of the PBMCs and permit expansion of effector T cells. Proliferation experiments attempted without the rest period led to no expansion of the PBMCs (data not shown). In summary, these data demonstrate that denileukin diftitox depletes Treg cells in vitro and enhances the proliferation of effector T cells, but that effector T cells may be affected by denileukin diftitox and require a rest period before they can become activated.
Enhanced antigen-specific T-cell reactivity of PBMCs after denileukin diftitox
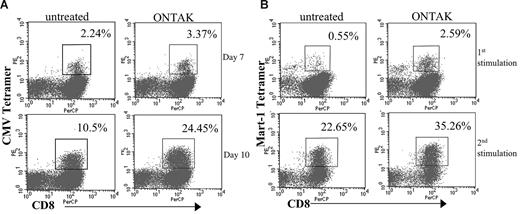
Because denileukin diftitox increased nonspecific T-cell proliferation, we also determined whether it could also enhance antigen-specific T-cell expansion. PBMCs from healthy donors were depleted of Treg cells using denileukin diftitox as described and cultured in vitro in the short term with CMVpp65 peptide. On days 7 and 10 of the CMVpp65 peptide–specific expansion, the percentage of CMVpp65 tetramer+ cells was determined for untreated and denileukin diftitox–treated cells (Figure 2A). PBMCs treated with denileukin diftitox showed a greater expansion of CMVpp65-specific T cells over untreated as shown by CMV tetramer staining at day 7 (3.4% vs 2.2%) and day 10 (24.4% vs 10.5%).
Expansion of PBMCs pretreated with denileukin diftitox by CMV and MART-1 peptides. PBMCs from a CMV+ donor were treated in vitro with denileukin diftitox or media for 18 hours. (A) Cells were replated in 10 U/mL IL-2 for 3 days and then cultured with CMVpp65 peptide and IL-2. Cells were analyzed by peptide–MHC tetramer staining on days 7 and 10 of culture. Data are presented as the percentage of CD8+CMV+ cells for untreated and denileukin diftitox–pretreated PBMCs. (B) Cells were replated in 10 U/mL IL-2 for 3 days and were then stimulated with MART-1 (26-35) peptide and IL-2. Peptide–MHC tetramer staining was performed on day 8 of culture. These cells were then restimulated with autologous PBMCs pretreated with denileukin diftitox (or media) as described, pulsed with MART-1 peptide, and irradiated. Cells were again analyzed by MART-1–specific tetramer 8 days after this second stimulation. We present the percentage of CD8+MART-1+ cells for untreated and denileukin diftitox–pretreated cells for both the first and second stimulations. Boxes on the graphs represent cells gated for analysis of percentage positive CD8 and tetramer.
Expansion of PBMCs pretreated with denileukin diftitox by CMV and MART-1 peptides. PBMCs from a CMV+ donor were treated in vitro with denileukin diftitox or media for 18 hours. (A) Cells were replated in 10 U/mL IL-2 for 3 days and then cultured with CMVpp65 peptide and IL-2. Cells were analyzed by peptide–MHC tetramer staining on days 7 and 10 of culture. Data are presented as the percentage of CD8+CMV+ cells for untreated and denileukin diftitox–pretreated PBMCs. (B) Cells were replated in 10 U/mL IL-2 for 3 days and were then stimulated with MART-1 (26-35) peptide and IL-2. Peptide–MHC tetramer staining was performed on day 8 of culture. These cells were then restimulated with autologous PBMCs pretreated with denileukin diftitox (or media) as described, pulsed with MART-1 peptide, and irradiated. Cells were again analyzed by MART-1–specific tetramer 8 days after this second stimulation. We present the percentage of CD8+MART-1+ cells for untreated and denileukin diftitox–pretreated cells for both the first and second stimulations. Boxes on the graphs represent cells gated for analysis of percentage positive CD8 and tetramer.
To demonstrate that Treg-cell depletion would enhance tumor antigen-specific T-cell responses, we analyzed the effects of denileukin diftitox on antigen-specific T-cell expansion with the melanoma antigen MART-1 peptide. PBMCs exposed to denileukin diftitox as described were stimulated with MART-1 peptide. The expanded PBMCs were then restimulated with autologous PBMCs that had again been treated with media alone or denileukin diftitox, pulsed with MART-1 peptide, and irradiated. The percentage of MART-1–specific T cells was determined by tetramer staining for both the initial and repeated stimulation showing an increase in MART-1–specific T cells in the denileukin diftitox–treated PBMCs (2.6% for denileukin diftitox–treated PBMCs compared with 0.55% MART-1+CD8+ T cells for the initial 8-day stimulation and 35.3% MART-1+CD8+ T cells compared with 22.5% for the second stimulation; Figure 2B). These data demonstrate that T-cell responses against self- and foreign recall antigens is enhanced after Treg-cell depletion with denileukin diftitox.
Analysis of the effect of Treg-cell depletion with denileukin diftitox in vivo: phase 1 clinical trial
Based on the preclinical data demonstrating that PBMCs depleted of Treg cells with denileukin diftitox exhibited enhanced immune responses, we performed a phase 1 clinical trial of a DC vaccine modified to express CEA, which was administered to patients with advanced CEA-expressing malignancies after denileukin diftitox administration in 2 different schedules (before the first dose of vaccine and before all 4 doses of the vaccine). Patients (9 men and 6 women aged 35 to 72 years) had colorectal cancer (n = 14) or breast cancer (n = 1); all had extensive metastatic disease but a performance status of 90% to 100% and had failed a median of 2 prior chemotherapeutic regimens. In the first cohort, 5 of 9 patients completed all 4 immunizations. In the second cohort, 5 of 6 patients completed all immunizations. Immunizations were well tolerated with only rare grade 3 (elevated alanine aminotransferase [ALT], aspartate aminotransferase [AST], and bilirubin attributed to disease progression and pleural effusion and dyspnea attributed to disease progression) and 1 grade 4 event (elevated creatinine attributed to initiation of an angiotensin-converting enzyme inhibitor).
Clinical course
In cohort 1, one of 9 patients experienced a minor response and one had stable disease, while the remainder had progressive disease at the end of immunizations. One of these patients with progressive disease later underwent a surgical resection of an enlarged retroperitoneal lymph node, and only necrotic tissue was identified. A second patient who initially had progressive disease was later found to have stabilization of disease on a subsequent CT scan. In cohort 2, all 6 patients had progression, but 1 experienced stability on subsequent CT scans for more than 6 months.
Effect of denileukin diftitox on peripheral blood CD4+CD25highFoxP3+ Treg cells
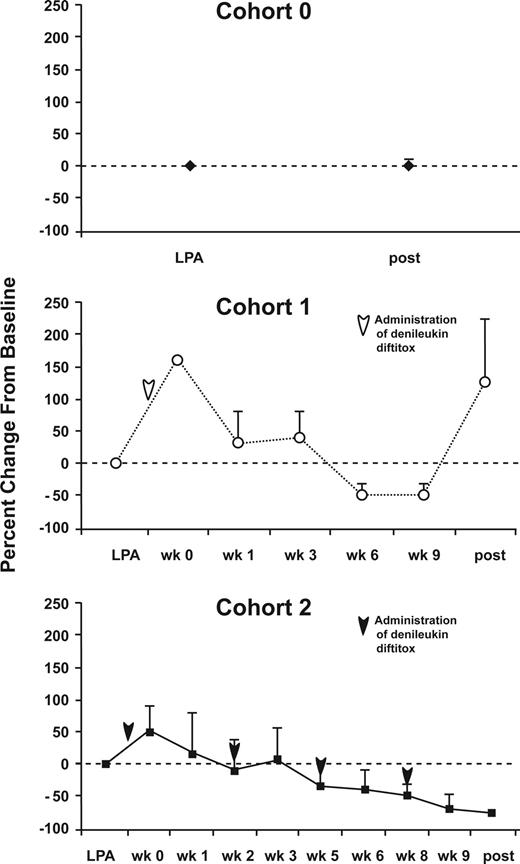
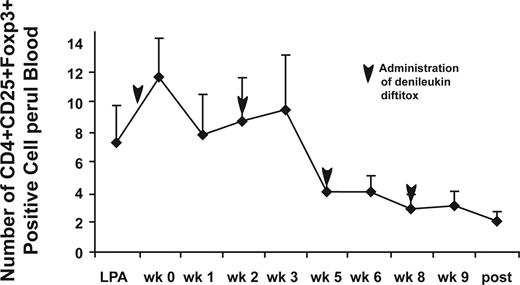
The circulating levels of CD4+CD25highFoxp3+ Treg cells were determined for both cohorts of this study as well as from our previous trial involving the DC+ rF-CEA(6D)-TRICOM vaccine (n = 5). This allowed a comparison of changes in Treg-cell levels after vaccinations alone (cohort 0), vaccinations after a single infusion of denileukin diftitox (cohort 1), and denileukin diftitox given before each vaccination (cohort 2; Figure 3). No change occurred in the percentage of CD4+CD25highFoxp3+ cells between the pre- and postvaccination samples in patients receiving the DC vaccine without denileukin diftitox (cohort 0). In cohort 1 (Figure 3B), although an initial increase is seen in the mean Treg-cell levels at week 1, the change in magnitude of CD4+CD25highFoxp3+ cells dips at weeks 6 and 9 before rebounding after the completion of the immunizations. Overall, only 2 of 5 patients who completed all immunizations in cohort 1 had a decreased percentage of Treg cells at the end of the immunizations. For the multidose cohort 2 (Figure 3C), the mean change in magnitude of CD4+CD25highFoxP3+ cells continues to decrease after the final vaccination. Overall, 4 of 4 patients in cohort 2 who completed all immunizations had a lower percentage of Treg cells at the end of all immunizations. For the second cohort, we also determined the total number of CD4+CD25highFoxp3+ cells per microliter of whole blood and found it to decrease beginning at week 5 and continue to decline after the final vaccination (Figure 4). These data indicate that repeated dosing of denileukin diftitox results in a consistent decrease in CD4+CD25highFoxP3+ Treg cells.
Changes in levels of Treg cells following immunization and/or administration of denileukin diftitox. PBMCs before and after vaccination for each cohort were analyzed by flow cytometry for expression of CD4, CD25, and Foxp3. (A) Cohort 0: PBMCs were collected before and after TRICOM-CEA vaccination. (B) Cohort 1: PBMCs were collected before TRICOM-CEA vaccination and denileukin diftitox administration (LPA), before vaccination and after denileukin diftitox (week 0), and weeks 1, 3, 6, 9, and following the final vaccine dose. (C) Cohort 2: before TRICOM-CEA vaccination and denileukin diftitox (LPA), before vaccination and after 1 dose of denileukin diftitox (week 0) and weeks 1, 2, 3, 5, 6, 8, 9, and following the final vaccination. Arrows indicate time of denileukin diftitox administration. The data are presented as the mean change in percentage of CD4+CD25+ cells that are 90% and greater Foxp3+ plus or minus SE for patients completing 4 vaccinations for each cohort (5 patients analyzed for each cohort). The data for each cohort are presented as the mean percentage change in the percentage of CD4+CD25highFoxp3+ cells from baseline, defined as the time of the leukapheresis (LPA) before any denileukin diftitox. Arrows indicate time of administration of denileukin diftitox.
Changes in levels of Treg cells following immunization and/or administration of denileukin diftitox. PBMCs before and after vaccination for each cohort were analyzed by flow cytometry for expression of CD4, CD25, and Foxp3. (A) Cohort 0: PBMCs were collected before and after TRICOM-CEA vaccination. (B) Cohort 1: PBMCs were collected before TRICOM-CEA vaccination and denileukin diftitox administration (LPA), before vaccination and after denileukin diftitox (week 0), and weeks 1, 3, 6, 9, and following the final vaccine dose. (C) Cohort 2: before TRICOM-CEA vaccination and denileukin diftitox (LPA), before vaccination and after 1 dose of denileukin diftitox (week 0) and weeks 1, 2, 3, 5, 6, 8, 9, and following the final vaccination. Arrows indicate time of denileukin diftitox administration. The data are presented as the mean change in percentage of CD4+CD25+ cells that are 90% and greater Foxp3+ plus or minus SE for patients completing 4 vaccinations for each cohort (5 patients analyzed for each cohort). The data for each cohort are presented as the mean percentage change in the percentage of CD4+CD25highFoxp3+ cells from baseline, defined as the time of the leukapheresis (LPA) before any denileukin diftitox. Arrows indicate time of administration of denileukin diftitox.
Number of Treg cells per microliter of blood after immunization and administration of denileukin diftitox for cohort 2. Whole blood was stained using BD Bioscience TruCount tubes analyzed by flow cytometry to determine total number of Treg cells per microliter of blood. The data are presented as the mean number of CD4+CD25highFoxp3+ cells/μL blood plus or minus SE for 5 patients completing 4 vaccinations for cohort 2. Analysis was performed before each vaccine injection and following the final vaccination, and before each denileukin diftitox infusion.
Number of Treg cells per microliter of blood after immunization and administration of denileukin diftitox for cohort 2. Whole blood was stained using BD Bioscience TruCount tubes analyzed by flow cytometry to determine total number of Treg cells per microliter of blood. The data are presented as the mean number of CD4+CD25highFoxp3+ cells/μL blood plus or minus SE for 5 patients completing 4 vaccinations for cohort 2. Analysis was performed before each vaccine injection and following the final vaccination, and before each denileukin diftitox infusion.
Immunologic analysis of CEA-specific T-cell response by ELISPOT assay
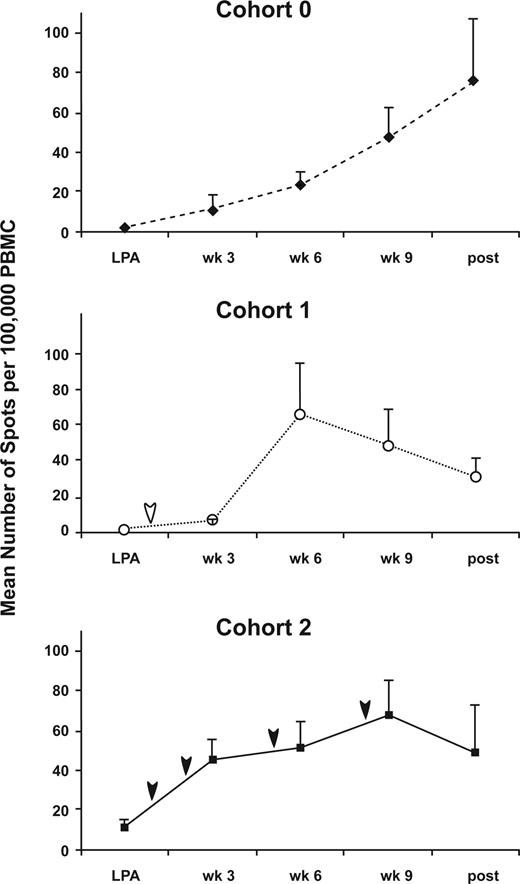
ELISPOT analysis was performed on freshly isolated PBMCs before each immunization and after the final immunization. Figure 5 demonstrates the mean number of IFNγ-producing cells per 100 000 PBMCs for each cohort in response to rF-CEA(6D)-TRICOM. Based on the definition of immune response proposed by the Immunotherapy Working Group,25 we found 13 of 14 evaluable patients had a CEA-specific immune response at some point during their immunizations for cohort 0, 4 of 5 for cohort 1, and 5 of 5 for cohort 2 (data not shown). Notably, denileukin diftitox was associated with an earlier time to first immune response (6.7 weeks for cohort 0, 5.25 weeks for cohort 1, and 3.6 weeks for cohort 2; P = .003 for cohort 2 vs cohort 0) and greater AUC of the ELISPOT response for cohort 2 (Tables 1, 2). The greater AUC indicates that there was a greater period of time that the immune response occurred in the multiple denileukin diftitox dose cohort 2.
Analysis of CEA-specific T cells by ELISPOT assay for each cohort.PBMCs collected before and after TRICOM-CEA vaccination were analyzed for their response to rF-CEA(6D)-TRICOM by ELISPOT assay. The data represent the mean number of IFNγ-producing cells per 100 000 PBMCs plus or minus SE for patients completing 4 injections for each cohort. Arrows indicate time of denileukin diftitox administration.
Analysis of CEA-specific T cells by ELISPOT assay for each cohort.PBMCs collected before and after TRICOM-CEA vaccination were analyzed for their response to rF-CEA(6D)-TRICOM by ELISPOT assay. The data represent the mean number of IFNγ-producing cells per 100 000 PBMCs plus or minus SE for patients completing 4 injections for each cohort. Arrows indicate time of denileukin diftitox administration.
AUC for ELISPOT and CFC assays
| Assay . | Cohort 0, n = 13 . | Cohort 1, n = 5 . | Cohort 2, n = 5 . |
|---|---|---|---|
| ELISPOT, median (range) | 177.98 (29.37-610.48) | 317.43 (76.12-780.8) | 532.05 (207.74-667.39) |
| CFC percentage of CD4, median (range) | 3.37 (1.28-8.17) | 3.8 (1.8-8.19) | 4.26 (3.02-13.16) |
| CFC percentage of CD8, median (range) | 6.03 (1.33-13.47) | 7.04 (2.81-9.63) | 13.25 (5.0-15.16) |
| Assay . | Cohort 0, n = 13 . | Cohort 1, n = 5 . | Cohort 2, n = 5 . |
|---|---|---|---|
| ELISPOT, median (range) | 177.98 (29.37-610.48) | 317.43 (76.12-780.8) | 532.05 (207.74-667.39) |
| CFC percentage of CD4, median (range) | 3.37 (1.28-8.17) | 3.8 (1.8-8.19) | 4.26 (3.02-13.16) |
| CFC percentage of CD8, median (range) | 6.03 (1.33-13.47) | 7.04 (2.81-9.63) | 13.25 (5.0-15.16) |
AUCs were computed based on ELISPOT measurements denoted as spots per 100 000 PBMCs times weeks and based on percentage of cytokine-secreting cells times weeks for the cytokine flow cytometry (CFC) assay.
Paired comparisons of AUCs among the 3 cohorts
| Assay . | Cohort 0 vs 1, P . | Cohort 0 vs 2, P . | Cohort 1 vs 2, P . |
|---|---|---|---|
| ELISPOT | .49 | .044 | .425 |
| CFC percentage of CD4 | .84 | .44 | .56 |
| CFC percentage of CD8 | .49 | .09 | .32 |
| Assay . | Cohort 0 vs 1, P . | Cohort 0 vs 2, P . | Cohort 1 vs 2, P . |
|---|---|---|---|
| ELISPOT | .49 | .044 | .425 |
| CFC percentage of CD4 | .84 | .44 | .56 |
| CFC percentage of CD8 | .49 | .09 | .32 |
Values determined by 2-sided Wilcoxon rank-sum test.
Immunologic analysis of CEA-specific T-cell response by intracellular cytokine staining
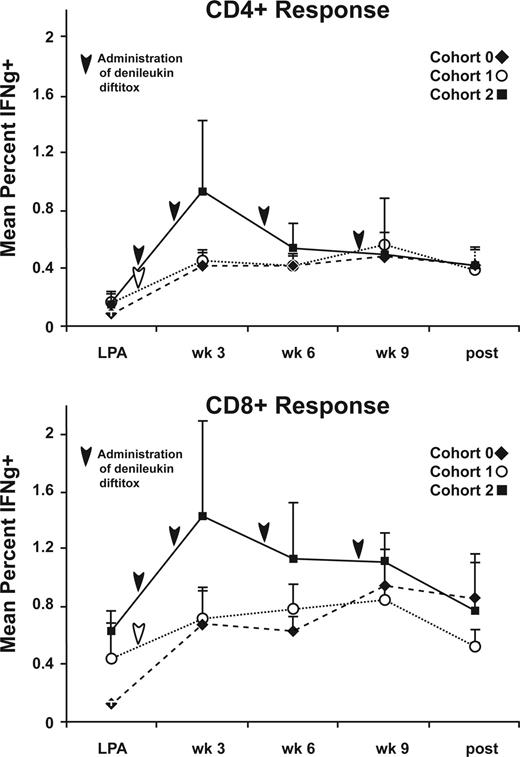
To characterize both CD4+ and CD8+ T-cell activation after immunization and/or denileukin diftitox infusion, we performed cytokine flow cytometry of freshly isolated PBMCs from each time point. We identified T cells responding to CEA exposure by the percentage of T cells that were CD69+ and producing IFNγ. Figure 6 depicts the mean percentage of CD4+IFNγ+ and CD8+IFNγ+ cells in response to rF-CEA(6D)-TRICOM stimulation before and after each immunization. Based on our experience in assessing positive and negative responses to various antigens,26 we chose a cut-off of 0.5% over background as indication of a positive response. We found that 14 of 14 patients had a CD4+ and CD8+ CEA-specific T-cell response at some point during their immunizations for cohort 0, 5 of 5 CD4+ response and 4 of 5 CD8+ response for cohort 1, and 5 of 5 CD4+ response and 4 of 5 CD8+ response for cohort 2 (data not shown). There were trends for an earlier CD4 and CD8+ T-cell response and for a greater AUC of the CD8+ immune response in cohort 2 (Tables 1,2), but these did not achieve statistical significance.
Cytokine flow cytometry results at each time point. PBMCs collected before and after TRICOM-CEA vaccination were analyzed for intracellular production of IFNγ in response to rF-CEA(6D)-TRICOM using flow cytometry. The data are presented as the mean percentage of CD4+ or CD8+CD69+IFNγ+ T cells for patients completing 4 injections for each cohort. Arrows indicate time of denileukin diftitox administration.
Cytokine flow cytometry results at each time point. PBMCs collected before and after TRICOM-CEA vaccination were analyzed for intracellular production of IFNγ in response to rF-CEA(6D)-TRICOM using flow cytometry. The data are presented as the mean percentage of CD4+ or CD8+CD69+IFNγ+ T cells for patients completing 4 injections for each cohort. Arrows indicate time of denileukin diftitox administration.
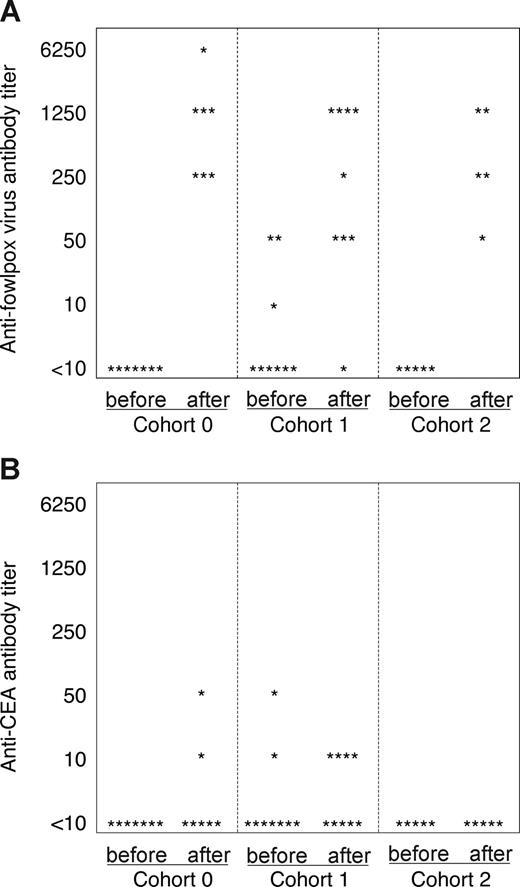
Serum antibody titers after vaccination
Analysis of antibody titers to both fowlpox and CEA antigen was performed on serum samples collected before and after immunization (Figure 7). Fowlpox-specific antibodies were detected in all patients tested in all cohorts after vaccination with the exception of one patient in the first cohort (Figure 7A). The titers ranged from 1:50 to 1:6250 among the responders, but were not greater in any of the cohorts. In contrast, CEA-specific antibodies, although of low titer, were more frequent in patients receiving a single dose of denileukin diftitox (cohort 1), but were not detected in any patient after multiple doses (cohort 2). These data suggest that tumor antigen-specific Treg cells may be more sensitive to the effects of denileukin diftitox than foreign antigen-specific Treg cells.
Fowlpox- and CEA-specific antibody induction for each cohort. Patient sera for each cohort were tested by ELISA for antibodies to (A) fowlpox virus and (B) CEA protein. The antibody titer for each patient tested is represented before and after vaccination by a star symbol.
Fowlpox- and CEA-specific antibody induction for each cohort. Patient sera for each cohort were tested by ELISA for antibodies to (A) fowlpox virus and (B) CEA protein. The antibody titer for each patient tested is represented before and after vaccination by a star symbol.
Discussion
This translational study was performed by first determining the effect of denileukin diftitox in vitro and then extending the observations into a phase 1 clinical trial. We observed that denileukin diftitox depleted Treg cells in vitro and resulted in enhanced immune reactivity. In the human clinical trial, we provide the first direct evidence that CD4+CD25highFoxP3+ Treg cells are depleted after multiple administrations of denileukin diftitox, but not after a single administration. CEA-specific T-cell responses were enhanced as measured by both ELISPOT and by cytokine flow cytometry, and these responses occurred earlier in the multiple dose cohort. Denileukin diftitox was not associated with an effect on primary/foreign antigen (fowlpox) antibody responses, but was associated with differences in antibody titer against the self-antigen, CEA.
Our study is unique because it directly assessed the level of circulating CD4+CD25highFoxP3+ Treg cells, evaluated 2 different dosing strategies, and compared the results with a group of patients not receiving denileukin diftitox. We also performed a complete immune evaluation (ELISPOT, cytokine flow cytometry, antibody responses, and Treg-cell phenotyping) at each immunization time point and thus have complete data permitting a better understanding of the time course of changes in immunity after denileukin diftitox–mediated depletion of Treg cells.
Our results on in vitro depletion of Treg cells is consistent with the report of Litzinger et al,22 who also observed that Treg cells decreased after denileukin diftitox treatment at 5 nM from 0.89% to 0.17% of CD4+ T cells. They observed little effect at 24 hours but a more marked effect 48 and 72 hours after the application of denileukin diftitox, whereas we saw an effect at 24 hours that became less pronounced after 72 hours. We suspect the differences in our observations are due to the fact that we cultured the PBMCs overnight (18 hours) with denileukin diftitox, whereas they applied it for only 2 hours. It is possible that the IL-2 portion of the denileukin molecule that persisted for a longer period of time in our experiment caused an activation of T cells to an express a Treg-cell phenotype on subsequent days of culture. Dannull et al18 cultured sorted Treg cells with denileukin diftitox for 6 hours and observed a decrease in Treg cells at 48 hours that was greater at 72 hours. It is possible that the lack of other PBMCs in their purified Treg-cell population did not permit the observation that other PBMCs could eventually develop a Treg-cell phenotype. Mahnke et al23 similarly noted killing of isolated CD4+CD25+ T cells at concentrations of 8 nM and greater denileukin diftitox after 24 hours of in vitro incubation. In contrast to our work, Attia et al27 reported no evidence of reduced Foxp3 expression in purified CD4+ cells exposed to up to 500 nM denileukin diftitox in vitro. Furthermore, the denileukin diftitox failed to remove cells capable of exerting a suppressive effect on effector cells. It is possible that differences in our results may be related to how FoxP3 was measured (we used flow cytometry and they used RT-PCR) and how the effect on effector T cells was measured.
Whether denileukin diftitox depletes Treg cells in vivo has been controversial. Dannull et al18 reported a decrease in Treg-cell percentage 4 days after a dose of 18 μg/kg per day of denileukin diftitox when enumerating the cells by CD4+CD25high expression. They also observed a decrease in FoxP3 copy number by RT-PCR. Mahnke et al23 reported that CD4+CD25+ Treg cells decreased for approximately 13 days after 18 μg/kg per day for 3 days of denileukin diftitox. The percentage of Treg cells decreased further after a second round of denileukin diftitox. In one patient who received 5 doses of denileukin diftitox, there was a slight decrease but never a complete depletion of Treg cells. Similar to our observation, they found some patients with a transient increase in Treg cells in the first few days of beginning the denileukin diftitox, but eventually all had a decrease in Treg cells. Barnett et al28 reported in a small pilot study that CD4+CD25+ T cells decreased after a 9- to 12-μg/kg dose of denileukin diftitox. Schonfeld and Mahnke24 reported that when patients were injected with a dose of 5 μg/kg denileukin diftitox, there was a reduction of CD4+CD25+ Treg cells in peripheral blood over a period of 2 weeks. However, when a dose of 18 μg/kg was used, no significant reduction of Treg cells occurred. These data were one of the reasons we started the second cohort at a lower dose of denileukin diftitox and may explain the better Treg-cell depletion observed in the multiple-dose group (cohort 2). In contrast, Attia et al27 reported no decrease in Treg cells. In their study, the denileukin diftitox was administered for 5 consecutive days every 21 days, which could possibly have resulted in stimulation of Treg cells by the IL-2 portion of the molecule. This report, using direct detection of CD4+CD25highFoxP3+ Treg cells, provides compelling evidence about the effects of denileukin diftitox in vivo.
A critical component of Treg-cell depletion is the effect on immune homeostasis. Mahnke et al23 reported vaccination with MART-1 and GP100 peptides following denileukin diftitox and observed an increase in ELISPOT and tetramer-positive T cells, but they did not have a control arm to determine whether the immune responses were enhanced by the denileukin diftitox. They argued that prior studies with peptides alone (without adjuvant) generally yielded very low immune responses otherwise. Dannull et al18 immunized patients with DCs loaded with tumor RNA either following denileukin diftitox or without any pretreatment and observed an improved stimulation of tumor-specific T-cell responses following denileukin diftitox compared with vaccination alone. Our study allows a direct comparison of the immune response with data obtained in our prior study using an identical vaccine in an identical patient population. In addition, the direct assessment of CD4+CD25highFoxP3+ Treg cells allowed us correlate Treg-cell depletion with enhanced immune response. Finally, assessment of the immune response to the neo-antigens found in the vaccine vector backbone allowed us to observe that there was specific enhancement of tumor antigen-specific immune responses but not general enhancement of immune responses.
The effect of the denileukin diftitox on antibody responses against CEA is surprising. It would appear that multiple doses have no effect on the antibody response to the foreign antigen (the fowlpox vector), but they cause a reduction in the self-antibody (CEA) response. Interestingly, Litzinger et al22 observed no effect of denileukin on the antibody response to foreign antigens (the vaccinia vector and influenza A [NP34]), but did not assess antibody responses to the tumor antigen CEA. One possibility for this observation is that denileukin diftitox could have direct inhibitory or destructive effects on tumor antigen-specific B cells. It is also possible that the apparent decrease in antibody against CEA, observed in our study, is due to random variation because the anti-CEA titers were low even in cohort 0 (the group not receiving denileukin diftitox).
In summary, denileukin diftitox eliminates Treg cells that are associated with an enhanced specific immune response to tumor antigen vaccination, and an earlier peak in T-cell response after multiple doses. Tumor antigen-specific antibody responses may be diminished after multiple doses. Future studies should better refine the number of doses and timing required to maximize the immune response activated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Liz Anderson and Emily Privette for healthy donor blood acquisition; Manar Ghanayem, Wiguins Etienne, Amanda Summers, and Karrie Comatas for generation of dendritic cells for the patient immunizations; Patti Frost for assistance with the statistical analysis; and Jill Boy for help with figures. We thank Sharon Peplinski for flow cytometry acquisition and analysis. We thank Eisai for supplying the denileukin diftitox (ONTAK) for the second cohort of the study. We also thank the National Cancer Institute Cancer Therapy Evaluation Program (CTEP) for supplying the rF-CEA(6D)-TRICOM.
This work was supported by grants from the National Institutes of Health (1R21-CA117126-01A1 to M.A.M. and 5POl-CA078673 to M.A.M. and H.K.L.).
National Institutes of Health
Authorship
Contribution: D.S., T.O., and A.C.H. performed experiments; A.C.H., T.O. and M.A.M. analyzed results and made the figures; H.K.L., M.A.M., and T.C. designed the research; A.C.H., H.K.L, T.C., and M.A.M. wrote the paper; and D.N. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Morse, Duke University Medical Center, Box 3233 MSRB 1 Rm 403, Durham, NC 27710; e-mail: morse004@mc.duke.edu.

![Figure 1. CD4+CD25highFoxP3+ Treg-cell levels and proliferation of PBMCs after in vitro treatment with denileukin diftitox. PBMCs from a healthy donor were treated with up to 8 nM denileukin diftitox or media alone (0 nM) for 18 to 20 hours and then cultured in media containing 10 U/mL IL-2 for 3 days. (A) Cells were stained for CD4, CD25, and Foxp3 expression on days 1 (after 18 hours of denileukin diftitox) and 4. The percentage of CD4+CD25highFoxp3+ cells are represented for each concentration. The percentage of CD4+CD25highFoxp3+ cells from freshly isolated PBMCs before the addition of denileukin diftitox is represented by a dashed line (1.82%). Data are representative of 3 repeated experiments. (B) PBMCs similarly depleted of Treg cells with denileukin diftitox and then rested in IL-2 for 3 days were stimulated in a proliferation assay with 0.5 μg/mL soluble OKT3 or media alone (unstimulated) for 4 days. Proliferation was determined by [3H]thymidine incorporation during a final 18 hours of culture, and the data are represented as mean cpm plus or minus SD. *P < .01 compared with 0 nM. Data are representative of 3 repeated experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/3/10.1182_blood-2008-01-135319/4/m_zh80160822500001.jpeg?Expires=1769093126&Signature=KFemFa0LyAAnzNlXg3MgTFpWWClKjalt-mzdE1YUBjoK9nnRS82Z~ZegVcoDLx56p1UA8hFQ3SCBJwZc3X2xzd1MFroSW6BwjvTsaujkbkWKIkm4ZuZuSWjOVDwXFkLHnbm-3Cj2jzMznnfddqjyth5ob6kyho8dZTCdVaTNIN~6zxzaySgeQR6Yj6AGPUsckih1hB-YpZacuB~zxaSMSRijXaR1fc7VPy8e68HKt~8SMb02ajAzB6bfhfo4~CLtgUtxUqPljzz44tQUeY0307oJgC8Xu3C7UHo~e-~s04QXoa4OVRjP6-IKkfP9aeaD7FhBKXMZ2zedOlIqdEWh9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal